Phase I study of taselisib in Japanese patients with advanced solid tumors or hormone receptor-positive advanced breast cancer
Funding information
Chugai Pharmaceutical Co., Ltd.
Abstract
Taselisib is a potent and selective phosphatidylinositide 3-kinase (PI3K) inhibitor. The present article reports the first study of taselisib administration in Japanese patients. The aim of this 2-stage, phase I, multicenter, open-label, dose-escalation study was to evaluate the safety, pharmacokinetics, and preliminary efficacy of taselisib as monotherapy in Japanese patients with advanced solid tumors (stage 1), and as part of combination therapy in Japanese patients with hormone receptor (HR)-positive locally advanced or recurrent breast cancer (stage 2). In stage 1, oral taselisib tablets 2, 4, and 6 mg/d were given in 28-day cycles. In stage 2, successive cohorts of patients received oral taselisib tablets (2 or 4 mg/d) with i.m. fulvestrant 500 mg. Nine and 6 patients were enrolled in stage 1 and stage 2, respectively. Taselisib was well tolerated. No dose-limiting toxicities were experienced in any cohort of patients and no deaths were observed. The most common treatment-related adverse events in stage 1 and stage 2, respectively, were rash (55.6%, 66.7%), diarrhea (44.4%, 66.7%), and stomatitis (44.4%, 66.7%). Taselisib was rapidly absorbed after dosage; its half-life was 12.9-32.0 hours in stage 1 and 16.1-26.5 hours in stage 2. Two patients achieved partial response (PR), 5 patients had stable disease (SD) and 2 patients had progressive disease (PD) in stage 1, and 1 patient had PR and 3 patients had SD in stage 2. All patients with PR were positive for PIK3CA gene mutations. These preliminary data suggest that taselisib may be effective in patients with PIK3CA-mutated solid tumors or HR-positive advanced breast cancer.
Abbreviations
-
- AE
-
- adverse events
-
- AUC
-
- area under the plasma-concentration time curve
-
- AUClast
-
- area under the plasma-concentration time curve from time 0 to time of last measurable concentration
-
- C max
-
- maximum plasma-concentration
-
- DLT
-
- dose-limiting toxicity
-
- ER
-
- estrogen receptor
-
- HR
-
- hormone receptor
-
- IHC
-
- immunohistochemistry
-
- ISH
-
- in situ hybridization
-
- PD
-
- progressive disease
-
- PFS
-
- progression-free survival
-
- PI3K
-
- phosphatidylinositide 3-kinase
-
- PR
-
- partial response
-
- PS
-
- performance status
-
- SD
-
- stable disease
-
- t max
-
- time to reach maximum concentration
1 INTRODUCTION
The PI3K pathway plays an important role in many cellular processes, including cell proliferation, differentiation, and survival.1, 2 The PI3K pathway is also involved in neoplastic transformations through mutation of one or more key molecules, including the class I isomers of the PI3K family (subunits p110α, p110β, p110γ, and p110δ).1 The PI3K/Akt/mammalian target of rapamycin (mTOR) pathway, which is downstream of the ER, is hyperactivated in patients who do not respond to ER-targeting therapy,3 and mutations in the PIK3CA gene are frequent.1 Mutations in p110α coding regions of the PIK3CA gene, so-called PI3Kα isoforms, are established in several solid tumors, and are particularly prevalent in cervical cancer, squamous cell lung cancer, head and neck cancer, and breast cancer.1 Therefore, small molecule inhibitors that target the PI3K pathway may provide clinical benefit in patients with these cancers.
Taselisib is an isoform-selective PI3K inhibitor that shows potent inhibitory activity against p110α, p110δ, and p110γ, but is ~30fold less potent against the p110β isoform.4 Preclinical studies have indicated that taselisib has increased antitumor activity in several PIK3CA-mutated tumor models, including uterine serous carcinoma,5 head and neck squamous carcinoma,4 and breast cancer.6
Breast cancer is the most common cancer diagnosed in women. In 2012, the estimated number of new breast cancer cases in women comprised 25% of all new cancer cases worldwide.7 Breast cancer is also the leading cause of death in women, accounting for 14.7% of the total deaths from cancer in 2012.7 Advances in endocrine therapy have resulted in the prolongation of both disease-free and overall survival for those patients with ER-positive (ER+) breast tumors; however, although 5-year recurrence rates have been significantly reduced with endocrine therapy, 5-year mortality rates have not decreased to the same degree.8 In addition, the response to endocrine agents is heterogeneous and 40%-50% of patients with ER+ tumors can relapse in the adjuvant setting.9 Prognosis of advanced/recurrent breast cancer is poor, with 5% of patients having PFS 10 years post-first chemotherapy dose.10, 11 Maintaining quality of life in these patients is an important part of their treatment, and therapeutic agents should be specific for relevant molecular targets and be used to treat appropriate patient populations in order to reduce AE.
This article presents the results of a phase I dose-escalation study conducted in Japan, which was designed to evaluate the safety, pharmacokinetics, and preliminary efficacy of taselisib monotherapy in patients with advanced solid tumors, and taselisib as part of combination therapy in patients with HR-positive locally advanced or recurrent breast cancer.
2 MATERIALS AND METHODS
2.1 Study design
This was a phase I, multicenter, open-label, modified 3 + 3 dose escalation study (JapicCTI-142630). This study was conducted in 2 stages: stage 1 evaluated the use of single and repeated doses of taselisib in Japanese patients with solid tumors, and stage 2 evaluated the use of taselisib with fulvestrant (Faslodex®) in Japanese patients with HR-positive locally advanced or recurrent breast cancer. Maximal doses of taselisib in stage 1 and stage 2 were planned as possible doses for use in future studies.
The study protocol was approved by Institutional Review Boards prior to patient recruitment and conducted in accordance with International Conference on Harmonization E6 Guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided written informed consent.
2.2 Patients
Japanese patients were included in the study if they were aged ≥20 years, had evaluable or measurable disease, a life expectancy of ≥12 weeks, an ECOG PS of 0 to 1, lesions that could be assessed by diagnostic imaging, adequate hematological and organ function and, for patients that were not treatment naïve, 2-4 weeks washout from previous cancer therapy prior to the start of taselisib dosing. For stage 1 of the study, patients were included if they had histologically or cytologically documented advanced or recurrent solid cancers, for which standard treatment is ineffective or for which there is no established standard treatment. For stage 2, patients were included only if they were postmenopausal women who were diagnosed with inoperable advanced or recurrent breast cancer. Patients had to have HR-positive disease by IHC and be HER2-negative on IHC and/or ISH of breast cancer tissue from primary tumors or from advanced or recurrent breast cancers. Patients were also required to have previously received endocrine therapy or were approved for initiating endocrine therapy.
Patients were excluded from any stage of the study if they had previous hypersensitivity to the excipients in taselisib tablets, diabetes mellitus requiring medication, central nervous system metastases, non-infectious bowel inflammation, or severe uncontrolled systemic cardiac, lung, or liver disease. Patients were also excluded from stage 2 of the study if they had received 2 or more previous chemotherapy regimens for advanced or recurrent breast cancer, had experienced previous hypersensitivity to components of fulvestrant, or had more than 1 primary cancer with a disease-free interval of less than 5 years. The PIK3CA mutation was detected by using an archived sample from the primary or recurrent tumor at the local site.
2.3 Study treatments
Study design and taselisib dosage procedures are summarized in Figures 1 and 2. In stage 1 of the study, taselisib monotherapy was given to successive cohorts of patients at escalating doses of 2, 4, and 6 mg/d (cohorts 1, 2, and 3, respectively) (Figure 1). Film-coated 2-mg tablets were used. Oral doses of taselisib were given once daily under fasting conditions, for 28-day cycles.12 Prior to starting the first cycle of treatment, patients received a single dose of taselisib, and day 1 of the first cycle was started 4-7 days after the single dose (Figure 2).
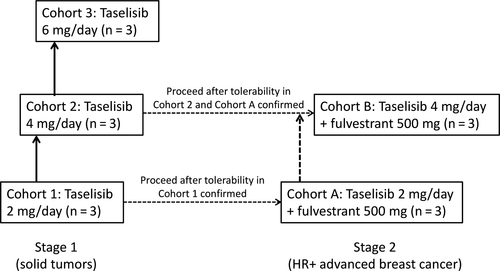
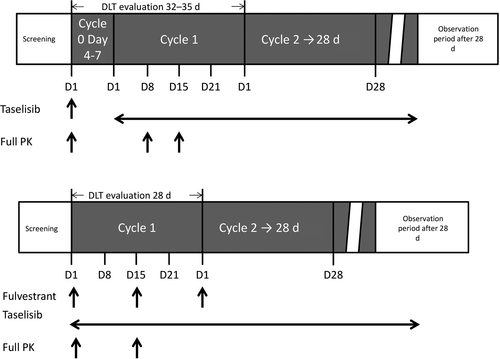
In stage 2 of the study, successive cohorts of patients received taselisib 2 or 4 mg/d in combination with fulvestrant 500 mg (cohorts A and B, respectively) (Figure 1). Similar to stage 1 of the study, taselisib was given orally once daily under fasting conditions and 1 cycle of treatment was 28 days. In stage 2, fulvestrant was injected i.m. before the taselisib dose on day 1 and day 15 of cycle 1, and day 1 of cycle 2 or later (Figure 2).
In both stages of the study, patients continued treatments until intolerable toxicity, PD, or withdrawal of consent.
2.4 Outcomes
Primary objectives of the present study were to evaluate the safety (including determining any DLT) and pharmacokinetics of taselisib.
DLT were set as determination factors to decide whether or not to proceed to the next cohort, and were defined as taselisib-related AE observed during the DLT evaluation period in each stage. The DLT evaluation period in stage 1 was from day 1 of cycle 0 to day 28 of cycle 1 (32-35 days) and the DLT evaluation period in stage 2 was from day 1 to day 28 of cycle 1 (Figure 2). The following were considered DLT: fasting grade 4 hyperglycemia, fasting grade 3 hyperglycemia persisting for ≥1 week after the start of treatment with oral diabetes medication, grade 4 fasting hypercholesterolemia or hypertriglyceridemia persisting for ≥2 weeks after the start of treatment with hyperlipidemia medication, grade ≥3 increased total bilirubin or alanine aminotransferase or aspartate aminotransferase, alkaline phosphatase, or any grade ≥3 non-hematological toxicity, with the exception of grade 3 diarrhea, nausea, vomiting, or skin toxicity. Hematological toxicities defined as a DLT included grade 4 thrombocytopenia or grade 4 neutropenia with fever lasting >5 days or accompanied by ≥38.0°C fever. The need to interrupt drug treatment for a total of 14 days or more during the DLT evaluation period because of AE was also considered a DLT.
Safety outcomes included the incidence, nature, and severity of AE (including DLT, laboratory test values, vital signs, and any other changes in patient condition considered clinically important). AE were graded according to the NCI Common Terminology Criteria for Adverse Events v4.0.
Plasma drug concentration-time data were used to calculate the following pharmacokinetic parameters of taselisib for both the single dose and repeated doses: Cmax, terminal t1/2, and AUC. AUClast was defined as the area from time 0 to time of last measurable concentration. During stage 1, plasma samples were collected during cycle 0 at predose, 0.5, 1, 2, 3, 4, 8, 24, 48, and 72 hours after dose, during cycle 1 at predose on day 1, at predose, 0.5, 1, 2, 3, 4, 8, and 24 hours after dose on day 8, at predose, 0.5, 1, 2, 3, 4, 8, and 24 hours after dose on day 15, and at predose on day 22, during cycle 2 or later cycles at predose on day 1, and on the last day of the study (Figure 2). During stage 2, plasma samples were collected during cycle 1 at predose, 0.5, 1, 2, 3, 4, 8, and 24 hours after dose on day 1, at predose, 0.5, 1, 2, 3, 4, 8, and 24 hours after dose on day 15, during cycle 2 and cycle 6 at predose on day 1, and on the last day of the study (Figure 2). Concentration of taselisib was determined using a validated liquid chromatography-tandem mass spectrometry analytical procedure, with a lower limit of quantification of 0.05 ng/mL.
The secondary objective of the study was to determine the preliminary efficacy of taselisib in both stages of the study. Efficacy was assessed by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.13 Assessment of lesions in stage 1 was conducted before the start of the study.
2.5 Statistical analysis
In both stages of the study, all statistical analyses were descriptive in nature; thus, no formal hypothesis testing was conducted. Efficacy was analyzed in the full analysis set, which comprised all patients who received at least 1 dose of study treatment. Safety was analyzed in the safety population, which comprised all patients who received at least 1 dose of treatment. All statistical analyses were carried out using SAS, version 9.2 (SAS Institute, Cary, NC, USA).
3 RESULTS
3.1 Participants
This study was conducted between 21 August 2014 and 17 May 2017. Overall, 9 patients were included in stage 1 and 6 patients were included in stage 2. Baseline characteristics of patients included in both stages of the study are reported in Table 1. In stage 1, all patients had a baseline ECOG PS of 0. PIK3CA mutations were found in 3 patients, including 1 PIK3CA E542K mutation and 2 PIK3CA H1047R mutations. In stage 2, 2 patients had baseline ECOG PS 1. PIK3CA mutations were found in 2 patients.
| Characteristic | Stage 1 | Stage 2 | |||||
|---|---|---|---|---|---|---|---|
| Taselisib | Taselisib 2 mg + fulvestrant (n = 3) | Taselisib 4 mg + fulvestrant (n = 3) | Total (n = 6) | ||||
| 2 mg (n = 3) | 4 mg (n = 3) | 6 mg (n = 3) | Total (n = 9) | ||||
| Median age, y (range) | 64 (64-72) | 67 (49-69) | 47 (45-56) | 64 (45-72) | 66 (55-70) | 67 (55-70) | 66.5 (55-70) |
| Sex, n (%) | |||||||
| Female | 2 (66.7) | 2 (66.7) | 3 (100) | 7 (77.8) | 3 (100.0) | 3 (100.0) | 6 (100.0) |
| Male | 1 (33.3) | 1 (33.3) | 0 | 2 (22.2) | 0 | 0 | 0 |
| ECOG PS, n (%) | |||||||
| 0 | 3 (100.0) | 3 (100.0) | 3 (100.0) | 9 (100.0) | 3 (100.0) | 1 (33.3) | 4 (66.7) |
| 1 | 0 | 0 | 0 | 0 | 0 | 2 (66.7) | 2 (33.3) |
| Cancer type, n (%) | |||||||
| Breast | 2 (66.7) | 2 (66.7) | 1 (33.3) | 5 (55.6) | 3 (100.0) | 3 (100.0) | 6 (100.0) |
| Thyroid | 1 (33.3) | 0 | 0 | 1 (11.1) | 0 | 0 | 0 |
| Cervical | 0 | 0 | 1 (33.3) | 1 (11.1) | 0 | 0 | 0 |
| Submandibular gland | 0 | 0 | 1 (33.3) | 1 (11.1) | 0 | 0 | 0 |
| Unknown | 0 | 1 (33.3) | 0 | 1 (11.1) | 0 | 0 | 0 |
| Histology, n (%) | |||||||
| Invasive ductal breast carcinoma | 2 (66.7) | 1 (33.3) | 0 | 3 (33.3) | 0 | 0 | 0 |
| Adenocarcinoma | 0 | 1 (33.3) | 0 | 1 (11.1) | 0 | 0 | 0 |
| Papillotubular carcinoma | 0 | 0 | 1 (33.3) | 1 (11.1) | 3 (100.0) | 3 (100.0) | 6 (100.0) |
| Mucoepidermoid carcinoma | 0 | 0 | 1 (33.3) | 1 (11.1) | 0 | 0 | 0 |
| Undifferentiated carcinoma | 1 (33.3) | 0 | 0 | 1 (11.1) | 0 | 0 | 0 |
| Squamous cell carcinoma | 0 | 1 (33.3) | 1 (33.3) | 2 (22.2) | 0 | 0 | 0 |
| Disease stage, n (%) | |||||||
| I | 1 (33.3) | 1 (33.3) | 1 (33.3) | 3 (33.3) | 0 | 2 (66.7) | 2 (33.3) |
| II | 0 | 0 | 1 (33.3) | 1 (11.1) | 1 (33.3) | 1 (33.3) | 2 (33.3) |
| III | 0 | 1 (33.3) | 1 (33.3) | 2 (22.2) | 0 | 0 | 0 |
| IV | 2 (66.7) | 1 (33.3) | 0 | 3 (33.3) | 2 (66.7) | 0 | 2 (33.3) |
| Primary tumor, n (%) | 0 | 1 (33.3) | 0 | 1 (11.1) | 1 (33.3) | 0 | 1 (16.7) |
| Known PIK3CA mutation, n (%) | 0 | 2 (66.7) | 1 (33.3) | 3 (33.3) | 0 | 2 (66.7) | 2 (33.3) |
| E542K | 0 | 0 | 1 (33.3) | 1 (11.1) | 0 | 0 | 0 |
| E545K | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (16.7) |
| H1047R | 0 | 2 (66.7) | 0 | 2 (22.2) | 0 | 0 | 0 |
| Other | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (16.7) |
| HER2 status by IHC, n (%) | |||||||
| 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 2 (66.7) | 3 (50.0) |
| 1+ | 0 | 0 | 0 | 0 | 2 (66.7) | 1 (33.3) | 3 (50.0) |
| Not done | 3 (100.0) | 3 (100.0) | 3 (100.0) | 9 (100.0) | 0 | 0 | 0 |
| HER2 status by ISH, n (%) | |||||||
| Negative | 0 | 0 | 0 | 0 | 0 | 3 (100.0) | 3 (50.0) |
| Not done | 3 (100.0) | 3 (100.0) | 3 (100.0) | 9 (100.0) | 3 (100.0) | 0 | 3 (50.0) |
| Hormone receptor ER+, n (%) | 0 | 0 | 0 | 0 | 3 (100.0) | 3 (100.0) | 6 (100.0) |
| Not done | 3 (100.0) | 3 (100.0) | 3 (100.0) | 9 (100.0) | 0 | 0 | 0 |
| Hormone receptor PGR+, n (%) | 0 | 0 | 0 | 0 | 3 (100.0) | 3 (100.0) | 6 (100.0) |
| Not done | 3 (100.0) | 3 (100.0) | 3 (100.0) | 9 (100.0) | 0 | 0 | 0 |
| Prior chemotherapy regimens, n (%) | |||||||
| 1 | 0 | 0 | 1 (33.3) | 1 (11.1) | 1 (33.3) | 0 | 1 (16.7) |
| 2 | 1 (33.3) | 0 | 0 | 1 (11.1) | 0 | 1 (33.3) | 1 (16.7) |
| 3 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (16.7) |
| 4 | 0 | 1 (33.3) | 1 (33.3) | 2 (22.2) | 0 | 2 (66.7) | 2 (33.3) |
| 5 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (16.7) |
| 8 | 2 (66.7) | 0 | 1 (33.3) | 3 (33.3) | 0 | 0 | 0 |
| 10 | 0 | 1 (33.3) | 0 | 1 (11.1) | 0 | 0 | 0 |
| 12 | 0 | 1 (33.3) | 0 | 1 (11.1) | 0 | 0 | 0 |
- ER, estrogen receptor; IHC, immunohistochemistry; ISH, in situ hybridization; PGR, progesterone receptor; PS, performance status.
3.2 Safety
Taselisib was well tolerated up to 6 mg as monotherapy in stage 1 and up to 4 mg as combination therapy in stage 2. No DLT were experienced in any cohort of patients and there were no deaths in the study of any cause. At least 1 treatment-related AE was experienced by all patients (100%) in stage 1 and in 83.3% of patients in stage 2 (Table 2). In stage 1, a total of 36 treatment-related AE occurred in 9 patients. Common treatment-related AE that occurred in 2 or more patients were rash (5 patients), diarrhea (4 patients), stomatitis (4 patients), decreased appetite (2 patients), and headache (2 patients). Of these treatment-related AE, grade ≥3 treatment-related AE were rash and diarrhea (1 patient each). A total of 5 serious AE (SAE) occurred in 3 patients: vertigo in 1 patient in cohort 2 which occurred 2 weeks after the last dose of taselisib and improved with supportive care, and erythema multiforme, rash, diarrhea, and lung infection in 2 patients in cohort 3. The patients discontinued taselisib as a result of these SAE in which the rash, diarrhea, and lung infection in cohort 3 were treatment-related. All patients with SAE recovered after discontinuation of treatment. In stage 2, a total of 32 treatment-related AE occurred in 5 patients. Common treatment-related AE that occurred in 2 or more patients were diarrhea (4 patients), stomatitis (4 patients), rash (4 patients), colitis (2 patients), and dry skin (2 patients). Of these treatment-related AE, grade ≥3 treatment-related AE were diarrhea and rash (1 patient each) and colitis (2 patients). Four SAE occurred in 3 patients in stage 2; they were 1 event of decreased appetite leading to discontinuation of treatment in 1 patient in cohort A, and 2 events of colitis and 1 event of gingivitis in 2 patients in cohort B. The patients discontinued treatment as a result of the colitis and recovered with corticosteroid. All of these SAE were considered treatment-related.
| Stage 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Taselisib | ||||||||
| 2 mg (n = 3) | 4 mg (n = 3) | 6 mg (n = 3) | Total (n = 9) | |||||
| All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | |
| Any TRAE, n (%)a | 3 (100.0) | 0 | 3 (100.0) | 1 (33.3) | 3 (100.0) | 2 (66.7) | 9 (100.0) | 3 (33.3) |
| Total no. AE | 6 | 0 | 16 | 1 | 14 | 4 | 36 | 5 |
| Rashb | 1 (33.3) | 0 | 2 (66.7) | 0 | 2 (66.7) | 1 (33.3) | 5 (55.6) | 1 (11.1) |
| Diarrhea | 0 | 0 | 2 (66.7) | 0 | 2 (66.7) | 1 (33.3) | 4 (44.4) | 1 (11.1) |
| Stomatitis | 1 (33.3) | 0 | 1 (33.3) | 0 | 2 (66.7) | 0 | 4 (44.4) | 0 |
| Decreased appetite | 0 | 0 | 1 (33.3) | 0 | 1 (33.3) | 0 | 2 (22.2) | 0 |
| Headache | 0 | 0 | 2 (66.7) | 0 | 0 | 0 | 2 (22.2) | 0 |
| Stage 2 | ||||||
|---|---|---|---|---|---|---|
| Taselisib 2 mg + fulvestrant (n = 3) | Taselisib 4 mg + fulvestrant (n = 3) | Total (n = 6) | ||||
| All grades | Grade ≥3 | All grades | Grade ≥3 | All grades | Grade ≥3 | |
| Any TRAE, n (%)a | 2 (66.7) | 1 (33.3) | 3 (100.0) | 3 (100.0) | 5 (83.3) | 4 (66.7) |
| Total no. AE | 13 | 2 | 19 | 6 | 32 | 8 |
| Diarrhea | 2 (66.7) | 1 (33.3) | 2 (66.7) | 0 | 4 (66.7) | 1 (16.7) |
| Stomatitis | 2 (66.7) | 0 | 2 (66.7) | 0 | 4 (66.7) | 0 |
| Rashc | 1 (33.3) | 0 | 3 (100) | 1 (33.3) | 4 (66.7) | 1 (16.7) |
| Colitis | 0 | 0 | 2 (66.7) | 2 (66.7) | 2 (33.3) | 2 (33.3) |
| Dry skin | 1 (33.3) | 0 | 1 (33.3) | 0 | 2 (33.3) | 0 |
- TRAE, treatment-related adverse event.
- a Patients could experience more than 1 episode of each TRAE.
- b Rash includes rash maculopapular.
- c Rash includes dermatitis acneiform.
3.3 Pharmacokinetics
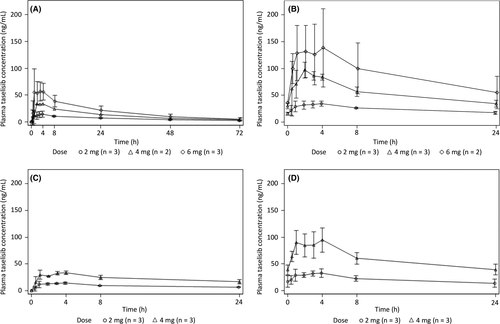
In stage 1, single and multiple oral doses of taselisib were rapidly absorbed with a tmax ranging from 0.483 to 8.00 hours (Table 3). Plasma concentration of taselisib gradually declined, with a t1/2 of 12.9-32.0 hours. Linearity was observed after both single and multiple doses of taselisib and no accumulation was found for multiple doses beyond the level estimated by the single dose (Figure 3). In stage 2, after single and multiple oral doses of taselisib plus fulvestrant 500 mg, taselisib was rapidly absorbed with a tmax ranging from 0.933 to 4.07 hours; similar to the pharmacokinetics observed in stage 1 of the study, the plasma concentration of taselisib then gradually declined, with a t1/2 of 16.1-26.5 hours (Table 3; Figure 3).
| Stage 1 | Stage 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taselisib | Taselisib 2 mg + fulvestrant (n = 3) | Taselisib 4 mg + fulvestrant (n = 3) | ||||||||
| 2 mg (n = 3) | 4 mg (n = 3) | 6 mg (n = 3) | ||||||||
| Day | Day 1 | Day 15 | Day 1 | Day 15 | Day 1 | Day 15 | Day 1 | Day 15 | Day 1 | Day 15 |
| Cmax, ng/mLa | 16.0 (9.45-24.8) | 34.5 (29.5-40.7) | 33.6c (30.6-36.8) | 102 (80.4-120) | 66.0 (40.9-106) | 110c (80.2-150) | 14.5 (12.3-17.5) | 35.3 (27.9-42.3) | 36.1 (34.2-37.4) | 93.2 (75.8-120) |
| tmax, hb | 3.97 (0.483-8.00) | 3.93 (1.00-4.00) | 4.02c (4.00-4.03) | 1.98 (0.500-2.00) | 2.05 (1.00-4.17) | 1.53c (0.983-2.07) | 2.98 (0.933-3.88) | 4.00 (0.967-4.03) | 3.03 (1.00-3.92) | 4.05 (4.02-4.07) |
| t1/2, ha | 28.1 (24.8-32.0) | NC | 26.9c (26.5-27.3) | 17.2 (15.7-19.6) | 22.4 (19.7-25.9) | 15.3c (12.9-18.1) | 20.6c (16.2-26.3) | NC | 20.6c (16.1-26.5) | NC |
| AUClast, ng·h/mLa | 431 (348-505) | 581 (511-651) | 875c (848-902) | 1300 (1120-1540) | 1330 (853-1780) | 1560c (1180-2060) | 216 (209-231) | 498 (394-693) | 550 (461-620) | 1380 (1140-1680) |
- AUClast, area under the plasma-concentration time curve from time 0 to time of last measurable concentration; Cmax, maximum plasma-concentration; t1/2, terminal half-life; NC, not calculated; tmax, time to reach maximum concentration.
- a Values are given as geometric mean (range).
- b Values are provided as median (range).
- c Values are from 2 patients.
3.4 Efficacy
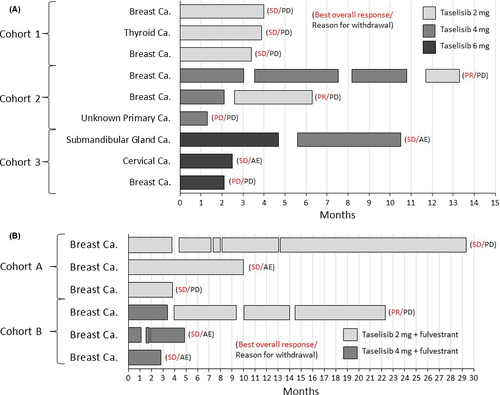
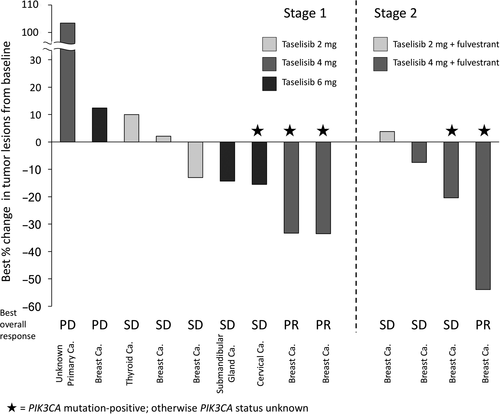
In stage 1, all 9 patients had target lesions and were eligible for assessment per RECIST 1.1 after starting taselisib treatment. Three patients continued the study treatment for ≥6 months (Figure 4A). The longest treatment duration was 13.3 months. PR was seen in 2 patients, SD was seen in 5 patients, and PD was seen in 2 patients, giving an overall response rate of 22% (2/9) and a disease control rate of 78% (7/9; Figure 5). The 2 patients with PR both had tumors with PIK3CA mutations (Figure 5), and the duration of response for the 2 patients with PR was 127 and 169 days, respectively. PFS was 38-398 days in stage 1. In stage 2, 4 patients had target lesions and were assessed after starting taselisib treatment. Two patients continued the study treatment for ≥12 months (Figure 4B). The longest treatment duration was 29.5 months. PR was observed in 1 patient and SD in 5 patients, giving an overall response rate of 17% (1/6) and a disease control rate of 100% (6/6; Figure 5). The 1 patient who achieved PR was positive for PIK3CA mutation (Figure 5), and the duration of response in this patient was 613 days. PFS was 110-884 days in stage 2.
4 DISCUSSION
This 3 + 3 dose-escalation study aimed to assess the safety, tolerability, pharmacokinetics, and preliminary efficacy of taselisib as monotherapy and in combination with fulvestrant in Japanese patients. Overall, the results showed good safety and preliminary efficacy. The results from stage 1 suggested that taselisib had good tolerability, with the common treatment-related AE being diarrhea, rash, stomatitis, headache, and decreased appetite; these were consistent with AE seen in the previous taselisib global Phase I study14 and studies of other PI3K inhibitors.15-18 All treatment-related SAE resolved or improved with dosage reduction, interruption, or discontinuation of treatment. Taselisib monotherapy was associated with 2 PR, all of which were in patients with PIK3CA-mutated tumors. Regarding the pharmacokinetics of taselisib monotherapy, taselisib was rapidly absorbed and gradually declined. Single-dose AUC and Cmax were dose-proportional and t1/2 was similar within the dose range of 2-6 mg. Dose proportionality was also observed in repeated doses. These results were consistent with the pharmacokinetics seen in the previous taselisib global Phase I study.14 The t1/2 of this study (range: 12.9-32.0 hours) was shorter than the t1/2 obtained in the previous study14 (mean: 36.7-43.8 hours) but this was because of the time points difference used for t1/2 calculation. There was a shorter t1/2 with repeated doses of taselisib monotherapy than with a single dose, although it should be noted that the t1/2 for the single dose was calculated from the concentration from 0 to 72 hours, whereas the t1/2 for the repeated dose was calculated from the concentration from 0 to 24 hours.
In stage 2, maximal dose of taselisib was planned up to 4 mg in combination with fulvestrant because the dose was selected based on efficacy and long-term safety in the global Phase I study and was evaluated in the Phase II part of the global Phase I/II study. Stage 2 results also showed good safety and preliminary efficacy of taselisib in combination with fulvestrant, particularly in the case of the patient who had a PR, whose duration of response was >600 days, suggesting promising efficacy for this combination. The common treatment-related AE observed in this stage of the study were diarrhea, stomatitis, rash, dry skin, and colitis, which are similar to the AE observed in stage 1 and in global Phase I studies of taselisib monotherapy14 and in studies of other PI3K inhibitors.15-18 All the treatment-related SAE were considered manageable and patients recovered or improved with appropriate treatment and interruption or reduction of taselisib dosing. Overall pharmacokinetic profile of taselisib, when given as a single dose or as repeated doses, was similar to that of taselisib monotherapy in this and other studies.14 These results suggest that the addition of fulvestrant to taselisib did not affect taselisib pharmacokinetics.
Fulvestrant is a selective ER degrader, and has been shown to improve PFS in postmenopausal patients with advanced breast cancer.19 Several studies of the combination of PI3K/Akt/mTOR inhibitors and fulvestrant have been completed (reviewed in19), and these generally show improved PFS, but an increased level of toxicity occurred when pan-PI3K inhibitors (such as pictilisib and buparlisib) were used in the combination.19 It is reasonable to expect that a more specific PI3K inhibitor would have less toxicity, consistent with the manageable toxicity observed with taselisib in this small study.
There are some limitations associated with the present study that may affect interpretation of the results. In the previous taselisib study,14 there was some indication that patients with PIK3CA mutations may have an increased response and, in the present study, the 3 PR were seen in patients with PIK3CA-mutated tumors; however, the small sample size of the present study and the unknown mutation status in the majority of patients means that the relationship between mutation status and efficacy cannot be completely determined. Based on the promising results of this study, further investigations into the safety, tolerability, and clinical efficacy of taselisib in larger patient populations are warranted.
In conclusion, this phase I study is the first study of taselisib tablet administration in Japanese patients. The data showed that taselisib has a good tolerability profile when given as monotherapy in doses up to 6 mg in patients with advanced solid tumors, and up to 4 mg in combination with fulvestrant when given to patients with HR-positive, HER2-negative advanced/recurrent breast cancer. PR were observed in 2 patients receiving taselisib monotherapy and in 1 patient receiving combination therapy. As all patients who had PR had PIK3CA-mutated tumors, taselisib is expected to be effective in patients with PIK3CA-mutated breast cancer.
ACKNOWLEDGMENTS
This study was supported by Chugai Pharmaceutical Co., Ltd (Tokyo, Japan). We thank all the participating patients and their families, as well as the investigators and clinical research coordinators. We would like to thank Yoshiko Okamoto, PhD, of inScience Communications for writing the outline, and Simone Boniface and Sheridan Henness, PhD, of inScience Communications, Springer Healthcare who wrote the first draft of the manuscript. This medical writing assistance was funded by Chugai Pharmaceutical Co., Ltd.
CONFLICT OF INTEREST
S. Takahashi has received honoraria from Daiichi-Sankyo, Sanofi, Bayer, Eisai, and research funding from Chugai, Taiho, MSD, Bayer, Novartis, AstraZeneca, Daiichi-Sankyo; C. Shimizu has received research funding from Chugai, MSD, Lilly and Pfizer; Y. Ito has received research funding from MSD, AstraZeneca, Novartis, Parexel, Chugai, Kyowa Kirin and Lilly; J. Tanaka, H. Kuriki, and Z. Gu are employees of Chugai Pharmaceutical Co., Ltd; H. Kuriki has stock ownership of Chugai Pharmaceutical Co., Ltd. The other authors have no conflicts of interest to declare.




