Podoplanin promotes progression of malignant pleural mesothelioma by regulating motility and focus formation
Funding Information
Program to Identify and Support Advanced Research on Drugs and Medical Devices of the National Institute of Biomedical Innovation (NIBIO), (Grant/Award Number:) Grant-in-Aids for Scientific Research in Innovative Areas, (Grant/Award Number:). Network for Integrative Research on Cancer Microenvironments of the Ministry of Education, Culture, Sports, Science, and Technology of Japan, JSPS KAKENHI Grant Number 16H05308
Abstract
Malignant pleural mesothelioma (MPM) is characterized by dissemination and aggressive growth in the thoracic cavity. Podoplanin (PDPN) is an established diagnostic marker for MPM, but the function of PDPN in MPM is not fully understood. The purpose of this study was to determine the pathogenetic function of PDPN in MPM. Forty-seven of 52 tumors (90%) from Japanese patients with MPM and 3/6 (50%) MPM cell lines tested positive for PDPN. Knocking down PDPN in PDPN-high expressing MPM cells resulted in decreased cell motility. In contrast, overexpression of PDPN in PDPN-low expressing MPM cells enhanced cell motility. PDPN stimulated motility was mediated by activation of the RhoA/ROCK pathway. Moreover, knocking down PDPN with short hairpin (sh) RNA in PDPN-high expressing MPM cells resulted in decreased development of a thoracic tumor in mice with severe combined immune deficiency (SCID). In sharp contrast, transfection of PDPN in PDPN-low expressing MPM cells resulted in an increase in the number of Ki-67-positive proliferating tumor cells and it promoted progression of a thoracic tumor in SCID mice. Interestingly, PDPN promoted focus formation in vitro, and a low level of E-cadherin expression and YAP1 activation was observed in PDPN-high MPM tumors. These findings indicate that PDPN is a diagnostic marker as well as a pathogenetic regulator that promotes MPM progression by increasing cell motility and inducing focus formation. Therefore, PDPN might be a pathogenetic determinant of MPM dissemination and aggressive growth and may thus be an ideal therapeutic target.
Malignant pleural mesothelioma (MPM) is a tumor that originates in the visceral pleura surrounding the lungs. This tumor then spreads to the lungs or into the thoracic cavity. The incidence of MPM is closely associated with asbestos exposure, and MPM can develop following a latent period of 20–40 years.1 Early detection of MPM is difficult, so curative resection is also difficult. Moreover, MPM has limited sensitivity to radiation therapy and cytotoxic chemotherapy and a very poor prognosis, so effective MPM treatments need to be developed.
We previously identified a type-I transmembrane sialoglycoprotein, podoplanin (PDPN, also known as Aggrus), as a platelet aggregating factor in highly metastatic tumor cells.2 PDPN binds to C-type lectin-like receptor 2 (CLEC2) expressed on platelets and causes platelets to aggregate; this aggregation depends upon Syk and Src family kinases and phospholipaseCγ2.3 As a result of its binding to CLEC2 on platelets, PDPN induces platelet aggregation and thereby promotes hematogenous metastasis.4 In addition, PDPN is known to form a complex with members of the ezrin-radixin-moesin (ERM) protein family, activate RhoA, and thus increase cell motility.5 PDPN is expressed by some non-cancer cells such as lymphendothelial cells and cancer-associated fibroblasts (CAF),6 though PDPN is frequently upregulated in several tumors, including squamous cell carcinoma, pleural mesothelioma, Kaposi's sarcoma, testicular germ cell tumors, and brain tumors.4, 7-9 PDPN is often expressed in MPM in particular, and the D2/40 antibody that recognizes PDPN is used as a marker of epithelial MPM.10
In the present study, we examined whether PDPN, a diagnostic marker for MPM, plays a critical role in disease progression.
Materials and Methods
Cell lines
The human mesothelioma cell lines MSTO-211H, H226, and H2452 were purchased from ATCC (Rockville, MD, USA). YMESO-14 cells were kindly donated by Dr. Y. Sekido (Aichi Cancer Research Center Institute, Nagoya, Japan) and EHMES-1 cells were kindly donated by Dr. H. Hamada (Hiroshima University, Hiroshima, Japan). NCI-H290 was provided by Dr. Adi F. Gazdar (University of Texas Southwestern Medical Center, Dallas, TX, USA). Cells were cultured in RPMI-1640 medium supplemented with 10% FBS (Life Technologies, Grand Island, NY, USA). All cell lines were tested and authenticated by the Japanese Cell Research Bank using short tandem repeat (STR) analysis and the GenePrint 10 System (Promega, Madison, WI, USA). Cells were regularly screened for Mycoplasma using a MycoAlert Mycoplasma Detection Kit (Lonza, Basel, Switzerland). ROCK inhibitors, Y-27632 and fasudil hydrochloride, were obtained from Wako Pure Chemical Industries (Osaka, Japan).
Western blotting
Lysates were prepared using Cell Lysis Buffer (Cell Signaling). The procedure for Western blotting was as previously described.11 The primary antibodies (Ab) used were anti- PDPN Ab (AngioBio Co.), anti-E-cadherin Ab (Cell Signaling), anti-N-cadherin Ab (Cell Signaling), anti-Vimentin Ab (Cell Signaling), anti-GAPDH Ab (Trevigen), and anti-β-actin Ab (Cell Signaling).
Cell viability assay
Cell viability was measured by the MTT dye reduction method. Tumor cells were plated onto 96-well plates at a density of 2 × 103/100 μL per well in RPMI 1640 plus 10% FBS and cells were incubated for 24 h. Drugs were then added to each well, and incubation was continued for another 72 h. Cell growth was measured with MTT solution (2 mg/mL; Sigma, St. Louis, MO, USA), as described previously.12
Wound healing assay
Cells were plated onto 6-well plates at 500 000 cells per well in RPMI 1640 plus 10% FBS and allowed to form a confluent monolayer. A wound was introduced by running a P200 pipette tip evenly across the monolayer. After incubation for 36 and 48 h, cells were observed using a microscope.
Transwell assay
Transwell assays were performed using the modified Boyden chamber method,13 with an 8-μm pore filter separating the upper and lower transwell chambers (BD Biosciences, NJ, USA). Tumor cells (104 cells/200 μL) were added to the upper chamber and incubated for 48 h. Cells that had not migrated were then removed from the upper surface of the filters with cotton swabs. Cells that had migrated to the lower surface of the filters were fixed, stained with H&E, and counted in six fields under a microscope at 200× magnification.
Transfection of the PDPN gene
Cells were seeded onto 6-well plates at a density of 1–2 × 105 cells/well. Twenty-four hours later, cells were transfected with a PDPN expression vector2 using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions. After treatment with neomycin (Sigma-Aldrich), cells were cultured in the presence of neomycin and clones expressing PDPN were isolated.
Small interfering RNA (siRNA) and short hairpin (sh) RNA for PDPN knockdown
shRNA was used to knock down PDPN. Lentiviruses were produced using 293T cells transfected with PCAG-HIV, CMV-VSV-G-RSV-Rev (RIKEN BioResource Center), and PDPN shRNA vectors (CS-H1-shRNA-EG; RIKEN BioResource Center).14 Transfection was performed using Lipofectamine 2000 reagent (Invitrogen, CA, USA) according to the manufacturer's instructions. The vector-containing medium was filtered through a 0.45-μm filter, and 8 μg/mL of Polybrene (SIGMA) was added for transduction of target tumor cells. siRNA was also used to knock down PDPN. Tumor cells were transfected with siRNAs against PDPN (Stealth siRNAs: HSS116395, HSS116397, HSS173792) or Stealth RNAi-negative control low GC Duplex #3 (Invitrogen) introduced into cells using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer's instructions.
RhoA-GTP binding assay
Direct activation of RhoA was measured using a G-LISA assay (Cytoskeleton Inc., CO, USA) according to the manufacturer's instructions. Briefly, the RhoA G-LISA kit used 96-well plates coated with the Rho-binding domain of the RhoA effector rhotekin. Rho-GDP was removed during washing steps and Rho-GTP was detected using a RhoA-specific antibody and chemiluminescence.
Orthotopic implantation
Tumor cells (1 × 106/100 μL) were injected into the thoracic cavity of SCID mice as reported previously.15 After the indicated periods, the mice were euthanized and tumor development was evaluated.
Immunohistochemistry
Formalin-fixed paraffin-embedded tumor sections were subjected to antigen retrieval and endogenous peroxidase blocking, and sections were incubated with primary antibody (Ab), anti-Ki-67 Ab (Dako), or anti-Yes-associated protein 1 (YAP1) Ab (Cell Signaling) at 4°C overnight. After incubating overnight, slides were rinsed and incubated with a peroxidase-labeled polymer. The tissue sections were then rinsed and stained with 3,3′-diaminobenzidine (DAB) substrate-chromogen and then counterstained with Hematoxylin Gill I (EMD Millipore) and bluing reagent (EMD Millipore).
Focus formation assay
Tumor cells were plated onto 6-well plates at 500 000 cells per well in RPMI 1640 plus 10% FBS and allowed to form a confluent monolayer. The confluent cell cultures were incubated for 14 days. The cultured cells were then stained with crystal violet and the foci were identified under a microscope.
Statistical analysis
The statistical significance of difference between the in vitro and in vivo data were analyzed by one-way anova using GraphPad Prism Ver. 4.01 (GraphPad Software, Inc., San Diego, CA, USA). Survival was analyzed by the Kaplan–Meier method. Differences between treatment and control groups were compared with the log-rank test. Differences at P < 0.05 were deemed significant.
Results
PDPN is highly expressed in pleural mesothelioma and promotes motility via RhoA/ROCK pathway activation
We first subjected tumors from 52 Japanese patients with MPM to immunostaining with the D2-40 antibody to determine if they expressed PDPN. Ninety percent of the tumors from Japanese patients with MPM tested positive for PDPN (Table S1, Fig. S1), so tumors from Japanese patients with MPM were found to express PDPN at high levels.
We then examined expression of PDPN in human MPM cell lines. High levels of expression were noted in three (H226, H2452, and EHMES-1) of six human MPM cell lines (Fig. 2a). In order to determine the role of PDPN in MPM cell lines, PDPN was knocked down with siRNA in 2 cell lines expressing high levels of PDPN (H226 and H2452). In H226 (Fig. 1b–d) and H2452 (Fig. S2) cells, knocking down PDPN did not alter cell viability but it did decrease cell motility. When PDPN was stably knocked down with shRNA in H226, motility was inhibited (Fig. 1e) but cell viability was not affected (Fig. S3c). The effects of shRNA were restored by transfection of shRNA-resistant PDPN mutants (Fig. S3a,b), so motility was definitely inhibited by knocking down PDPN.
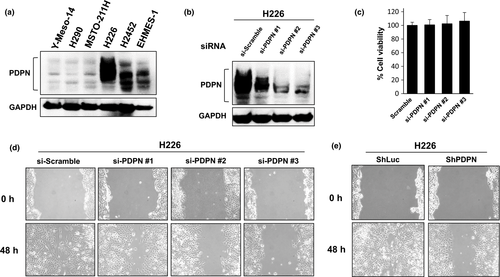
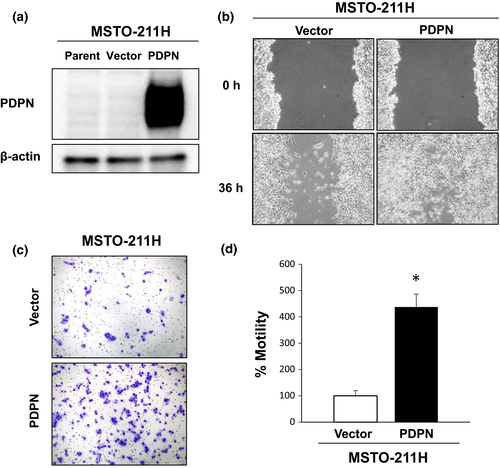
In contrast, transfection of PDPN into MSTO-211H cells expressing low levels of PDPN (Fig. 2a) did not alter cell viability (data not shown) but it did enhance motility. Both a wound healing assay and a migration assay using a transwell system revealed enhanced motility as a result of overexpression of PDPN (Fig. 2b–d). These findings revealed that PDPN regulates the motility of MPM cells.
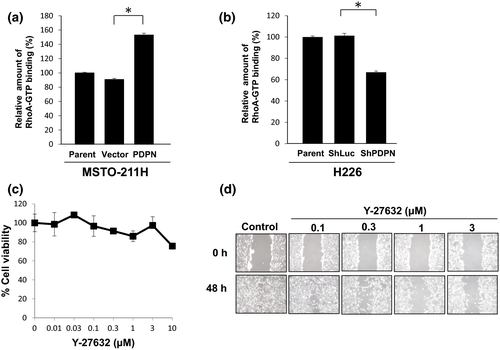
Podoplanin is known to bind to the ERM protein family and activate Rho.5 Thus, we examined whether or not PDPN promotes the motility of MPM cells via the Rho/ROCK/Rac pathway. Transfection of PDPN into MSTO-211H cells resulted in increased RhoA-GTP binding (Fig. 3a). Conversely, knocking down PDPN with specific siRNA in H226 cells resulted in decreased RhoA-GTP binding (Fig. 3b). We also explored the effects of compounds that inhibit ROCK downstream of Rho. Y-27632 is widely used as a ROCK inhibitor, and fasudil hydrochloride has been clinically approved for treatment of delayed cerebral vasospasms following a subarachnoid hemorrhage since it inhibits ROCK.16 Neither Y-27632 nor fasudil hydrochloride altered the viability of MSTO-211H/PDPN cells (Fig 3c, Fig. S4a), but the two ROCK inhibitors did inhibit motility in a dose-dependent manner (Fig. 3d, Fig. S4b). These findings indicate that PDPN activates the RhoA/ROCK pathway, thus promoting the motility of MPM cells.
PDPN promotes the progression of mesothelioma in the orthotopic implantation model
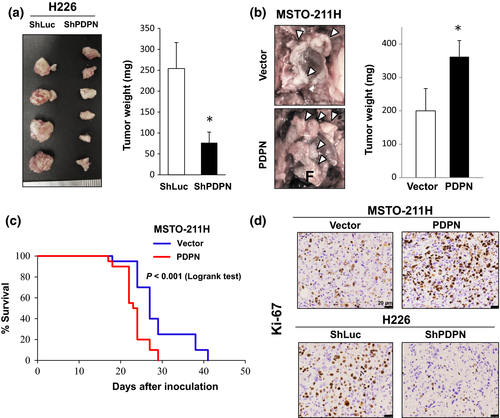
The effects of PDPN on tumor progression were examined in a model of orthotopic intrathoracic implantation in SCID mice. In H226 cells, tumor progression (the intrathoracic tumor burden) was inhibited by the knockdown of PDPN with shRNA (Fig. 4a). In contrast, tumor progression was promoted by transfection of PDPN into MSTO-211H cells, and mice had a significantly reduced survival time (Fig. 4b,c). Similarly, tumors produced by MSTO-211H cells transfected with PDPN had an increased number of Ki-67-positive proliferating cells. In contrast, tumors produced by H226 cells when PDPN was knocked down with shRNA had a reduced number of Ki-67-positive proliferating cells (Fig. 4d, Fig. S5). In another cell line expressing low levels of PDPN (H290), transfection of the PDPN gene resulted in enhanced tumor progression in a model of orthotopic implantation and an increased number of Ki-67-positive proliferating cells (Fig. S6). However, PDPN expression did not affect the engraftment rate or the number of tumors produced by MPM cells. These findings revealed that PDPN sustains the growth of MPM cells in vivo and that it promotes tumor progression in the thoracic cavity.
PDPN promotes focus formation in vitro and induces YAP1 activation associated with a low level of E-cadherin expression in vivo
Promotion of MPM cell motility by PDPN may not be the only factor responsible for tumor enlargement in vivo. Therefore, we focused on contact inhibition as another mechanism. Loss of contact inhibition is a strong indicator of cell transformation17 and facilitates tumor progression. We performed a focus formation assay to examine the effect of PDPN on contact inhibition in MPM cells. PDPN blocked contact inhibition and promoted the formation of foci in MSTO-211H (Fig. 5a) and H290 (Fig. S7) cells. In contrast, knockdown of PDPN enhanced contact inhibition in H226 cells (Fig. 5b) resulting in a remarkable decrease in the number of foci.
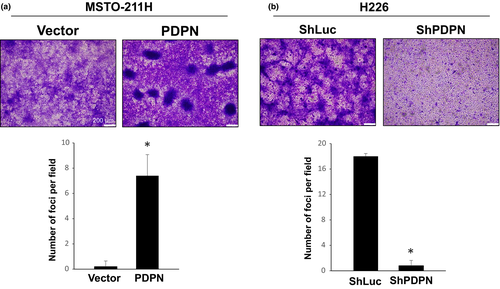
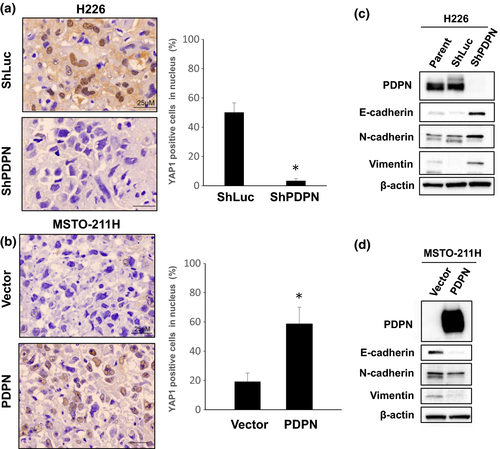
YAP1 is reported to block contact inhibition and promote tumor progression.18 In order to determine the mechanisms by which PDPN blocks contact inhibition, YAP1 expression was examined in tumors obtained from an orthotopic implantation model. YAP1 is a transcription factor that facilitates the transcription of various genes upon nuclear translocation.18 In tumors produced by H226 cells expressing high levels of PDPN, YAP1 was detected in the nuclei of 50% or more tumor cells, indicating that YAP1 was activated. In tumors produced by H226 cells upon PDPN knockdown with shRNA, YAP1 was not detected in the nuclei of most tumor cells, indicating that YAP1 was inactive (Fig. 6a). In tumors produced by MSTO-211H or H290 cells that express low levels of PDPN, YAP1 was not detected in the nuclei of most tumor cells. In tumors produced by MSTO-211H or H290 cells transfected with PDPN, YAP1 was detected in the nuclei of 60% or more tumor cells (Fig. 6b, Fig. S8). Moreover, PDPN knockdown in H226 cells resulted in increased E-cadherin expression, whereas transfection of PDPN into MSTO-211H cells resulted in decreased E-cadherin expression (Fig. 6c,d). These findings suggest that PDPN blocks contact inhibition via decreased expression of E-cadherin and YAP1 activation.
Discussion
The monoclonal antibody D2-40 recognizes PDPN, which is a well-established diagnostic marker for MPM. In the present study, we demonstrated that PDPN stimulates motility of MPM cells via activation of the RhoA/ROCK pathway. Moreover, PDPN blocks contact inhibition and it promotes progression of MPM in the thoracic cavity. These findings clearly indicate that PDPN plays a major role in the progression of MPM.
Podoplanin increased the motility of MPM cells in both cells natively expressing high levels of PDPN and in cells that were forced to express PDPN by gene transfection. These findings agree with the results of Yamaki et al.,19 which were obtained by forced expression of PDPN in MPM cells. Increased motility due to PDPN was noted in various types of cells, including breast cancer cells, pancreatic beta cell carcinoma,20 and cells derived from the kidney.21 This view is uncontested, but there is still debate as to whether motility increased by PDPN occurs via induction of an epithelial–mesenchymal transition (EMT) or not.20, 21 In the present study, PDPN expression in MPM cells did cause a decrease in E-cadherin expression but it did not necessarily trigger an increase in vimentin (Fig. 6c,d). Moreover, PDPN expression did not induce a typical morphological change to mesenchymal-like spindle-shaped cells (data not shown). These findings suggest that PDPN may activate the RhoA/ROCK pathway and increase the motility of MPM cells, even if a classical EMT is not induced. Whether or not increased motility due to PDPN occurs via induction of a classical EMT (associated with a decrease in E-cadherin and an increase in vimentin) may differ depending on the type of cancer.
Loss of contact inhibition is a hallmark of cell transformation.22 Recent studies have reported that loss of contact inhibition involves activation of YAP1. YAP1 is a transcription coactivator downstream of the Hippo pathway. Activation of the Hippo pathway inhibits cell growth and induces cell death. If the Hippo pathway is inactivated, however, YAP1 is translocated to the nucleus, where it facilitates the transcription of various factors and promotes cell growth.23 In addition to the role of YAP1 in the Hippo pathway, YAP1 activity is also regulated by E-cadherin.24 E-cadherin is reported to regulate contact inhibition in proliferating breast cancer cells by directly controlling YAP localization.25 The current study found that expression of PDPN in MPM cells caused decreased expression of E-cadherin, it promoted the nuclear translocation of YAP1, and it caused a loss of contact inhibition. In the future, analysis of the mechanisms by which PDPN inhibits expression of E-cadherin should prove crucial to revealing the full scope of the mechanisms by which PDPN blocks contact inhibition.
Malignant pleural mesothelioma usually originates in the visceral pleura and then spreads into the thoracic cavity, where it rapidly grows. In the present study, we found that PDPN blocks contact inhibition resulting in increased focus formation and increased motility in MPM cells. These findings suggest that PDPN may be a regulatory factor that plays a key role in facilitating the enlargement of the primary tumor, its dissemination, or the growth of implants. Moreover, PDPN is a potent platelet-aggregating factor.2, 4 Recent studies have noted that platelets were present in tumor tissue and that various growth factors released by the aggregated platelets promoted the growth of cancer cells in tissue.26 Thus, PDPN expressed on MPM cells may promote the growth of MPM both by blocking contact inhibition in tumor cells and by causing the aggregation of platelets that have leaked into the thoracic cavity and their release of platelet-derived growth factor.
Podoplanin is a diagnostic marker for MPM and it promotes the progression of MPM, so PDPN could potentially serve as a therapeutic target. Over the past few years, anti-PDPN antibodies that inhibit platelet aggregation and mediate antibody-dependent cellular cytotoxicity (ADCC) have been created to target PDPN.27-33 Agents that target PDPN should prove effective in treating MPM.
In conclusion, we demonstrated that PDPN, a well-established diagnostic marker for MPM, plays a major role in MPM progression by stimulating cell motility via RhoA/ROCK pathway activation and by blocking contact inhibition associated with decreased E-cadherin expression and YAP1 activation. Collectively, our findings indicate that PDPN is an ideal target for treatment of patients with MPM.
Acknowledgments
The authors wish to thank Yoshitaka Sekido (Aichi Cancer Center) and Hamada (Hiroshima University) for providing the human mesothelioma cell lines used in this study. This study was supported in part by grants (to SY and NF) from the Program to Identify and Support Advanced Research on Drugs and Medical Devices of the National Institute of Biomedical Innovation (NIBIO), by Grant-in-Aids for Scientific Research in Innovative Areas (to SY and NF) as part of the “Network for Integrative Research on Cancer Microenvironments” of the Ministry of Education, Culture, Sports, Science, and Technology of Japan, JSPS KAKENHI Grant Number 16H05308 (to SY).
Disclosure Statement
The authors have no conflicts of interest in this study.
Abbreviations
-
- CAF
-
- cancer-associated fibroblasts
-
- CLEC2
-
- C-type lectin-like receptor 2
-
- ERM
-
- ezrin-radixin-moesin
-
- MPM
-
- malignant pleural mesothelioma
-
- PDPN
-
- podoplanin
-
- SCID
-
- severe combined immune deficiency
-
- Sh
-
- short hairpin
-
- YAP1
-
- Yes-associated protein 1




