Novel pegylated interferon-β as strong suppressor of the malignant ascites in a peritoneal metastasis model of human cancer
Funding Information
Japan Science and Technology Agency, Japan Agency for Medical Research and Development.
Abstract
Malignant ascites manifests as an end-stage event during the progression of a number of cancers and lacks a generally accepted standard therapy. Interferon-β (IFN-β) has been used to treat several cancer indications; however, little is known about the efficacy of IFN-β on malignant ascites. In the present study, we report on the development of a novel, engineered form of human and murine IFN-β, each conjugated with a polyethylene glycol molecule (PEG-hIFN-β and PEG-mIFN-β, respectively). We provide evidence that these IFN-β molecules retain anti-viral potency comparable to unmodified IFN-β in vitro and manifested improved pharmacokinetics in vivo. Interestingly, PEG-mIFN-β significantly inhibited the accumulation of ascites fluid and vascular permeability of the peritoneal membrane in models of ovarian cancer and gastric cancer cell xenograft mice. We further show that PEG-hIFN-β directly suppresses VEGF165-induced hyperpermeability in a monolayer of human vascular endothelial cells and that PEG-mIFN-β enhanced gene expression for a number of cell adhesion related molecules in mouse vascular endothelial cells. Taken together, these findings unveil a hitherto unrecognized potential of IFN-β in maintaining vascular integrity, and provide proof-of-mechanism for a novel and long-acting pegylated hIFN-β for the therapeutic treatment of malignant ascites.
Malignant ascites is a complex condition caused by intraperitoneal tumor metastasis and vascular hyperpermeability.1, 2 Patients of this ailment present with symptoms of abdominal swelling, pain and nausea, and often suffer disability and a reduced quality of life. While paracentesis can alleviate symptoms associated with ascites, there are no established options to control or treat underlying mechanism(s) of the illness.1, 2 As such, there is an unmet medical need for additional treatment options.
Type I interferons (IFN) are a family of soluble proteins that act pleiotropically but are known principally for their regulation of the immune system.3, 4 Over the past several decades IFN have received considerable attention for the treatment of various cancers.4 Of their many effects on tumors, IFN have been implicated in the activation of immune cell populations, including natural killer (NK) cells, enhancement of cross-priming activity, and inhibition of regulatory T cells’ (Tregs’) suppressive function.5, 6 IFN have also been described to directly inhibit tumor cell proliferation and disrupt the intratumoral microvasculature.7 Nevertheless, the direct administration of type I IFN to cancer patients has failed to deliver solid clinical outcomes as was anticipated by preclinical studies. As a result, the enthusiasm surrounding this once promising therapeutic had waned.
In recent years, interest in type I IFN, particularly IFN-β, for the treatment of cancers has returned owing to a close association with newer biologic-based immunotherapies.4 In this context, a recent study of T-cell therapy using a CD19-targeting chimeric antigen receptor (CAR) demonstrated that an integrated CD28 and 4-lBB intracellular signaling exhibited a more robust anti-tumor effects via activation of the IRF7/IFN-β pathway.8 Furthermore, IFN-β was also shown to augment the anti-tumor activity of an anti-PD-1 checkpoint blockade by further decreasing the number of intratumoral Treg cells and increasing expression of PD-1 on effector cells in the tumor microenvironment through the activation of tumor-associated macrophages.9 IFN-β also gained attention in the context of STING (stimulator of interferon genes)-mediated induction of antitumor T cells by cytosolic DNA.10 These findings may suggest that IFN-β exerts effective anti-tumor activity when intratumoral IFN levels are sustained in the tumor microenvironment through local secretion.
Does IFN-β signaling affect malignant ascites? Little is known regarding whether and how IFN-β can suppress accumulation of ascites. In previous clinical studies, IFN-β was examined for palliative treatment of malignant ascites caused by refractory ovarian or gastric cancers; however, no promising results were obtained even with a daily high-dose injection.11-13 Because IFN-β has a short half-life and is rapidly cleared from the circulation in vivo,14 we asked whether a stable and long-acting form of IFN-β could address this question.
In this study, we developed a stabilized IFN-β that retained potency and enabled us to evaluate the potential of IFN-β to suppress ascites formation. Both a pegylated human IFN-β (PEG-hIFN-β) and pegylated murine IFN-β (PEG-mIFN-β), each carrying a single 43-kDa branched polyethylene glycol (PEG) molecule at a lysine residue were engineered. Administration of the PEG-mIFN-β in a murine tumor model showed significant suppression of ascites accumulation, without significant inhibition of tumor volume. Interestingly, administration of PEG-mIFN-β inhibited peritoneal vascular hyperpermeability in peritoneal metastasis mice. We also found that PEG-hIFN-β significantly inhibited VEGF165-induced vascular endothelial cell (EC) hyperpermeability, using HUVEC monolayer. Consistently, PEG-mIFN-β, but not unmodified mIFN-β, showed a sustained expression of cell adhesion-related genes in mouse vascular EC.
On the basis of these results, we have revealed a previously unrecognized facet of IFN-β action on vascular permeability and present a potential clinical benefit for utilization of PEG-hIFN-β for the treatment of malignant ascites and other related diseases.
Materials and Methods
A GCIY cell suspension (1 × 107 cells in 0.5 mL/mouse) in cold HBSS(−) was transplanted intraperitoneally into male KSN/Slc mice (7 or 8 weeks of age).15 Ascites was collected from the individual mice at 5 weeks after the transplantation of the GCIY cell as an initial ascitic paracentesis. The ascitic paracentesis was performed by puncturing the abdominal cavity with a winged intravenous needle (18-gauge) under isoflurane anesthesia and collecting ascites through the needle. The PEG-hIFN-β, PEG-mIFN-β or PEG-IFN-β combination (c-PEG-IFN-β), which was a mixture of PEG-hIFN-β and PEG-mIFN-β, was administered intraperitoneally or subcutaneously at doses such that PEG-hIFN-β and PEG-mIFN-β were each administered in an amount of 10 000 or 300 000 units/mouse. Administration was initiated on the same day as the initial ascitic paracentesis and performed on alternate days for three or six times in total. In the control group, instead of PEG-IFN-β, the same volume of 20 mmol/L acetate buffer (pH 4.5) containing 150 mmol/L NaCl was administered in the same manner. Re-accumulated ascites after the initial ascitic paracentesis was collected from individual mice in each group under anesthesia on the day following the final administration. Individual mice whose ascites had been collected were killed under isoflurane anesthesia or by cervical dislocation. Tumor that had metastasized to the peritonium was removed with forceps and scissors. An OV-90 cell suspension (5 × 106 cells/mouse) in cold PBS(−) was transplanted intraperitoneally into female SCID mice (8 weeks of age). Ascites was collected from the individual mice 6 weeks after the transplantation of the cell suspension. The ascitic paracentesis and administration of PEG-IFN-β were performed as described above. The weight of ascites (g) was measured, and individual mice in which ascites accumulation of 0.1 g or more was observed were subjected to the following experiment. After administrations of PEG-IFN-β three times, mice were killed by exsanguination under isoflurane anesthesia and subjected to laparotomy for ascites and tumor collection. The survival rate of each group from the day on which administration was initiated to 12 days thereafter was examined. Other materials and methods are described in detail in Data. S1.
Results
The generation of a novel form of human and mouse pegylated interferon-β and their antiviral activity in vitro
We first developed a new form of PEG-hIFN-β that contains a single PEG molecule (43 kDa) covalently linked to the amino group of lysine (Lys) 134 amino acid of recombinant human IFN-β produced in Escherichia coli. PEG-mIFN-β was conjugated with a single PEG molecule linked to Lys 134 (see Materials and Methods). To demonstrate that PEG-hIFN-β and PEG-mIFN-β retained potency, highly purified proteins were tested in an in vitro antiviral assay. As shown in Figure 1a, PEG-hIFN-β showed more potent anti-viral activity than unpegylated hIFN-β-1b in a cytopathic effect (CPE) assay using vesicular stomatitis virus (VSV) and Sindbis virus from human amnion origin cells (FL cells); hIFN-β-1b, referred to as Betaseron/Betaferon, is a stable formulation of recombinant hIFN-β that lacks the N-terminal Met residue and has a Cys17 to Ser mutation, and is currently in clinical use.16 Similarly, potent antiviral activity of PEG-mIFN-β against Sindbis virus was also confirmed on murine fibroblast L cell-derived LY cells (Fig. 1b). Notably, both human and mouse PEG-IFN-β proteins showed significantly more potent anti-viral activity than their respective unpegylated IFN-β molecules, likely owing to their prolonged stability, as discussed further below.
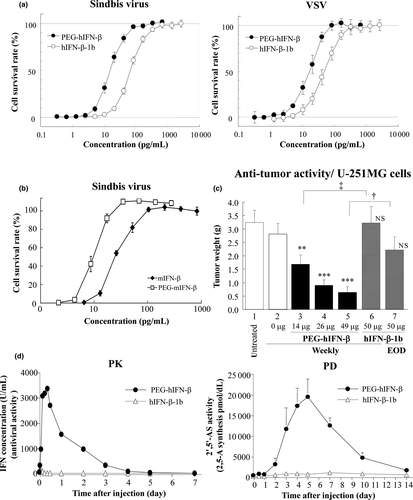
Anti-tumor activities of human and mouse pegylated interferon-β molecules
We next examined PEG IFN-β for its potential to augment anti-tumor activity. First, PEG-hIFN-β was examined in a U251 MG cell xenograft mice tumor model. As shown in Figure 1c, the weekly administration of PEG-hIFN-β reduced tumor weight beginning at 4 weeks in a dose-dependent manner. In contrast, unpegylated IFN-β showed little, if any, anti-tumor activity even following every-other-day administration (Fig. 1c).
We reasoned that this enhanced anti-tumor activity elicited by PEG-hIFN-β is due to its prolonged stability and potency in vivo. Therefore, we first examined pharmacokinetics (PK) and pharmacodynamics (PD) relationships in rabbit. As shown in Figure 1d, PEG-hIFN-β elicited a dramatically prolonged anti-viral activity as compared to unpegylated hIFN-β, as measured by CPE assay. This was accompanied by a sustained, robust induction of 2′–5′-oligoadenylate synthetase (2′-5′OAS). The PK of PEG-mIFN-β was also examined in the mouse. Following subcutaneous administration of PEG-mIFN-β, serum concentration of PEG-mIFN-β increased dose-dependently, and reached Cmax 3 h after administration (Fig. S1a). The estimated half-life was approximately 40-fold longer than that of unpegylated mIFN-β, indicating that pegylation of mIFN-β also markedly improved retention in circulation (Table S1).
Thus, these results demonstrate the superiority of PEG-hIFN-β over unpegylated IFN-β. Of note, the PEG-hIFN-β markedly reduced anti-IFN antibody responses in the mouse and leukocyte infiltration at injection site in the rabbit as compared with unpegylated hIFN-β (Fig. S1b,c). This is perhaps due to “masking” of PEG-hIFN-β by pegylation at Lys 134, as this region corresponds to an epitope on unpegylated hIFN-β for neutralizing antibody responses reported during the treatment of patients.17 The reduced immunogenicity represents another beneficial aspect of this PEG-hIFN-β molecule for its clinical use.
Suppression by pegylated interferon-β of malignant ascites accumulation in mice
The anti-tumor activities observed upon administration of the PEG-hIFN-β prompted us to examine the effect of PEG-hIFN-β on ascites formation. Human epithelial ovarian cancer OV-90 cells were inoculated in SCID mice18 and then administered PEG-IFN-β. Because it is not clear whether PEG-IFN-β would act on the host or cancer cells, the tumor-inoculated mice were treated with a combination of PEG-hIFN-β and PEG-mIFN-β (hereafter referred to as c-PEG-IFN-β). The c-PEG-IFN-β was subcutaneously administered three times every other day and treatment was initiated on the same day as the initial ascitic paracentesis. Interestingly, expansion of abdominal circumference with ascites accumulation was suppressed in mice treated with c-PEG-IFN-β (Fig. 2a). In addition, a significant difference in survival was noted between the untreated and the c-PEG-IFN-β-treated groups (Fig. 2b).
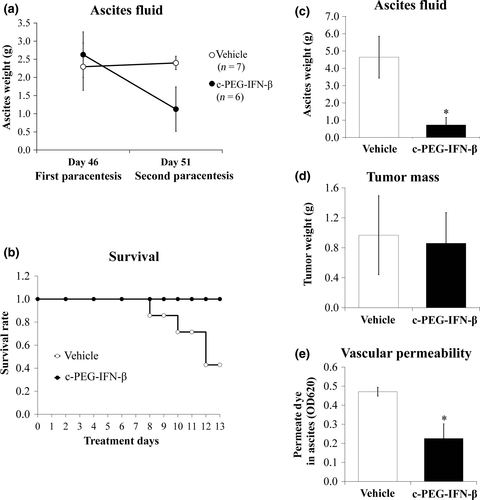
We next examined whether the c-PEG-IFN-β therapy suppresses both malignant ascites accumulation and tumor weight using Foxn1nu mice inoculated with GCIY cells.15 As shown in Figure 2c, c-PEG-IFN-β again showed a robust suppressive effect on ascites formation as compared with control mice. In contrast, no significant difference was found in the intraperitoneal tumor weight between the c-PEG-IFN-β-treated and untreated mice (Fig. 2d).
Because ascites is exuded from peritoneal blood vessels, particularly capillaries or microvessels, we next examined peritoneal vascular hyperpermeability in these mice. As expected, there was a marked reduction in the passage of Evan's blue dye into the peritoneal cavity of c-PEG-IFN-β-treated mice as compared with vehicle-treated mice (Fig. 2e). Thus, c-PEG-IFN-β-mediated suppression of ascites accumulation is likely to be the consequence of the inhibition of vascular hyperpermeability.
Suppression by pegylated interferon-β of peritoneal membrane vascular hyperpermeability is independent of its anti-tumor activity
We hypothesized that it is PEG-mIFN-β within c-PEG-IFN-β that suppresses ascites accumulation through its inhibition of vascular hyperpermeability in the mouse. To further address this we next treated GCIY cell-inoculated nude mice with PEG-hIFN-β or PEG-mIFN-β and c-PEG-hIFN-β following development of ascites. As shown in Figure 3a, PEG-mIFN-β-treated mice showed a reduced amount of permeated Evan's blue dye in the peritoneal cavity, which was comparable to c-PEG-IFN-β, while such effect was marginal following PEG-hIFN-β treatment.

We then examined the degree of ascites fluid accumulation and vascular permeability in OV-90 cell-inoculated SCID mice and found that ascites weight and dye permeation into the peritoneal cavity were both significantly decreased by treatment with PEG-mIFN-β as compared to vehicle treatment (Fig. 3b,c). As expected, we did not observe any significant differences in the peritoneal tumor weight between vehicle and PEG-mIFN-β treatment (Fig. 3d). Given that the ascites fluid of PEG-mIFN-β-treated mice not only had a lower volume but also less hemorrhage, we were prompted to measure the effect of treatment on the number of erythrocytes in the ascites. As shown in Figure 3e, PEG-mIFN-β-treated mice showed a marked reduction of erythrocytes in their ascites as compared with vehicle-treated mice. These results also suggest that PEG-mIFN-β precludes macromolecule leakage into the peritoneal cavity.
Effect of pegylated interferon-β on VEGF-induced hyperpermeability of vascular endothelial cells
Vascular hyperpermeability is triggered by tumor cell-derived factors, such as VEGF, and malignant ascites formation is associated with elevated serum or ascites levels of VEGF.17, 19, 20 VEGF alters the permeability of vascular EC in the peritoneal membrane21 and, therefore, plays a role in the pathogenesis of ascites. To examine a direct effect of PEG-IFN-β on VEGF-induced hyperpermeability of vascular EC in vitro, we analyzed VEGF165 -induced permeability using a HUVEC monolayer model (Fig. S2). As shown in Figure 4a, PEG-hIFN-β significantly inhibited VEGF-induced hyperpermeability at a relatively low concentration and in a dose-dependent manner. We also found that the inhibitory effect of PEG-hIFN-β on VEGF165-induced permeability is dependent on treatment time, reaching a maximum level at 48 h after PEG-hIFN-β addition (Fig. 4b).
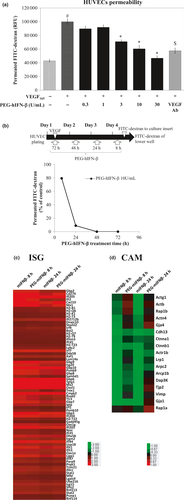
It has been reported that IFN-β treatment on vascular EC causes a decrease of permeability through the induction of the ecto-5-nucleotidase CD73.22 Therefore, we examined the effect of PEG-hIFN-β on the expression of CD73 on HUVEC and found a marked induction of CD73 molecule in a dose-dependent and time-dependent manner (Fig. S3a). We also found that both PEG-IFN-β and natural IFN-β induced CD73 expression by intermittent treatment, however, the induction level was significantly lower in natural IFN-β as compared to PEG-IFN-β (Fig. S3b), indicating that the PEG-IFN-β is more potent in suppressing ascites retention and endothelial cell permeability. In addition, we found that the induced CD73 expression is dependent of the continuous presence of PEG-IFN-β treatment (Fig. S3c). We further examined the contribution of CD73 on the reduced permeability using AMPCP, a specific CD73 inhibitor, and found that the PEG-hIFN-β-mediated inhibition of permeability is suppressed by AMPCP, albeit partially (Fig. S4). These results suggest that PEG-hIFN-β suppresses VEGF-induced hyperpermeability of EC in a CD73-dependent and CD73-independent manner.
Induction of cell adhesion related genes by pegylated interferon-β in vascular endothelial cells
To gain further insight into the effect of PEG-IFN-β to inhibit vascular permeability, we performed a microarray analysis to examine mRNA expression levels of cell adhesion molecule-related genes (CAM) in EC from mouse lung. As anticipated from the above results, while no significant differences were observed between PEG-mIFN-β and mIFN-β for IFN-stimulated gene (ISG) expression levels at 8 h after the ligand stimulation of EC, PEG-mIFN-β stimulation resulted in the sustained expression of ISG even at 24 h after treatment (Fig. 4c). Interestingly, PEG-mIFN-β stimulation of EC enhanced mRNA expression for α-actinin, β-actin, connexin37, rap1 and zo-2 gene, or retained the mRNA expression levels of CAM as compared with control EC (Fig. 4d). In contrast, the effect of mIFN-β was not as remarkable as that of PEG-mIFN-β (Fig. 4d). Therefore, these findings offer a mechanism underlying the novel activity of PEG-mIFN-β, not endowed with mIFN-β; namely, inhibition of tumor-induced accumulation of ascites fluid by the enhanced expression of genes associated with the maintenance of peritoneal vascular hyperpermeability.
Discussion
In this study, we first engineered a novel biologics solution by conjugating a polyethylene glycol molecule at the Lys 134 of IFN-β, termed PEG-IFN-β. In addition to retaining anti-viral activity in vitro, PEG-IFN-β demonstrated prolonged pharmacodynamics in vivo. As a result, PEG-IFN-β showed more potent anti-tumor activity as compared to unconjugated IFN-β.
PEG-hIFN-β shows decreased immunogenicity in mice and rabbits as compared with hIFN-β or hIFN-β-1b, likely the result of modification to Lys 134, which is located on the carboxyl terminal side of the fourth helix domain of hIFN-β and is the predicted IFNAR2 binding site (Fig. S1b,c).23 Recombinant IFN-β treatment has been shown to induce secretion of antibodies against IFN-β, which block its binding to IFNAR or neutralize specific activity in multiple sclerosis patients.24 Of note, antibodies against IFN-β typically recognize residues 1–12, 121–132 and 151–162 as an epitope, which is juxtaposed with the Lys 134 residue.17 Therefore, we propose that pegylation of Lys 134 restricts antibody access to these epitopes and, consequently, decreases the immunogenicity of the therapeutic. In addition to the pre-clinical study, a phase 1 clinical trial was conducted with Japanese healthy male volunteers to assess the PK and PD, and the safety and tolerability of single subcutaneous doses of PEG-hIFN-β.25
Our study reveals a new aspect of IFN-β as a therapy for the treatment of malignant ascites mediated through a direct effect to reduce vascular permeability in mice with peritoneal membrane metastases. Although IFN-β has multifunctional effects to enhance anti-tumor activity in vivo, little is known about the efficacy of IFN-β on malignant ascites. We demonstrated that the PEG-mIFN-β “monotherapy” significantly suppressed the accumulation of ascites, accompanied with a decrease in vascular permeability of the peritoneal membrane in mice inoculated with human cancer cells (Fig. 3b–d). Because IFN-β has species-specific biological activity and murine IFN-β does not show cross-activity on human cancer cells in human cancer xenograft mice,26 our results indicate that PEG-mIFN-β suppresses the increased microvascular permeability of the peritoneal membrane and, consequently, inhibits formation of malignant ascites.
We also demonstrated that PEG-hIFN-β directly inhibited VEGF-induced hyperpermeability in vascular EC in a time-dependent and dose-dependent manner (Fig. 4b). Our data suggest that this inhibition is due, at least in part, to the induction of CD73 in EC (Figs S3a and S4). Furthermore, our data suggest that continuous stimulation by PEG-hIFN-β is required for the maximum induction of CD73 expression (Fig. S3c). These data in toto indicate that the prolonged activity endowed with PEG-hIFN-β has an advantage in the suppression of malignant ascites fluid accumulation as compared with the short-acting unmodified hIFN-β.
Previously, another PEG-IFN-β, called PEG-IFN-β-1a, was generated that contains 20-kDa PEG at the N-terminal region.27 However, this form of PEG-IFN-β totally loses its activity when PEG with 40 kDa is conjugated.27 Another IFN-β-1b bound with 40-kDa PEG also exhibited attenuated anti-viral activity to 19–35%, compared with non-pegylated IFN-β-1b.28 Furthermore, the IFN-β-1b molecule itself has reduced anti-viral activity compared to natural IFN-β.16 It is generally known that the molecular weight of PEG conjugated to a protein molecule correlates with its prolonged action;29, 30 hence, although careful comparison would be required, we envisage that the newly generated IFN-β carrying 43-kDa PEG at Lys 134 may be superior over the 20-kDa PEG-IFN-β and 40-kDa PEG-IFN-β-1b in many aspects. Although continuous injection of the natural form of IFN-β may have similar therapeutic effect as the use of PEG-IFN-β, it is beneficial for patients to avoid frequent injections. In regards to safety, PEG-IFN-β-1a showed similar adverse effects compared with the non-pegylated form.31
Furthermore, PEG-IFN-β may suppress vascular endothelial hyperpermeability through upregulation of CAM and improve the dysregulated intercellular contacts in EC. We found that PEG-mIFN-β specifically increased CAM, including α-actinin, β-actin, connexin 37, rap1a, rap1b and zo-2 genes in EC from mouse lung (Fig. 4d). It has been shown that both α-actinin and β-actin are regulatory factors of the actin cytoskeleton and play a role in stable cell adhesion through their binding to α-catenin protein.32 Connexin 37 is a membrane protein integral to gap junction of EC and maintains the normal vascular function.33, 34 Rap1 contributes cross-linking of cortical actin with VE-cadherin, and promotes stable cell–cell contacts.35, 36 It has been shown that EC monolayers lacking Rap1 have increased permeability in EC.37 Zo-2, known as tight junction protein Tjp2, is a peripheral membrane scaffolding protein and could be upregulated by angiopoietin-1, which counteracts VEGF-induced hyperpermeability.33, 38 These upregulated genes are positively associated with the strength of cell–cell interaction. Thus, PEG-IFN-β can exert stabilization of the cell adhesion signals in the multiple ways and restore the excessive permeability to the normal state.
Why does PEG-IFN-β enhance the expression of these genes but not unmodified IFN-β? The prolonged action or the different receptor-binding mode of PEG-IFN-β may contribute to the unique gene expression profiles. Thus, it is possible that unconjugated IFN-β has potential to induce these genes but the induction is too low to be detected by the above analyses. Obviously, further work will be required to clarify the detailed mechanism of PEG-IFN-β-induced increase and maintenance of CAM in EC.
In conclusion, the present study offers a new therapeutic basis for the treatment of malignant ascites, for which a generally accepted standard therapy is not available, with a pegylated IFN-β protein due to its effects on vascular permeability. In addition, PEG-IFN-β may also be considered as a replacement for the treatment of other types of disease conditions by IFN-β and other type I IFN therapies given the potential to reduce the frequency of administration and immunogenicity, and the increased efficacy.39, 40
Acknowledgments
We thank M. Sato, N. Tanaka, Y. Yamashita, Y. Nakada and R. Horiuchi for their help with producing and evaluating PEG-hIFN-β/PEG-mIFN-β. This work was partially supported by the “A-STEP Contract Development” program of the Japan Science and Technology Agency (JST) and the Japan Agency for Medical Research and Development (AMED). The Department of Molecular Immunology at the University of Tokyo is supported by BONAC Corporation and Kyowa Hakko Kirin.
Disclosure Statement
Tomokatsu Iwamura, Hideki Narumi, Tomohiko Suzuki, Katsuyuki Mori, Koji Yamashita, Yoshiaki Tsushima, Tomomi Asano, Akiko Izawa, Shinobu Momen, Kazumi Nishimura, Hiromi Tsuchiyama, Masashi Uchida, Yuji Yamashita, and Kiyoshi Okano are employees of Toray Industries. This work was partially supported by the “A-STEP Contract Development” program of the Japan Science and Technology Agency (JST) and the Japan Agency for Medical Research and Development (AMED).




