Preclinical and first-in-human phase I studies of KW-2450, an oral tyrosine kinase inhibitor with insulin-like growth factor receptor-1/insulin receptor selectivity
Funding Information:
Kyowa Hakko Kirin Co., Ltd and Kyowa Hakko Kirin Pharma, Inc.
Abstract
Numerous solid tumors overexpress or have excessively activated insulin-like growth factor receptor-1 (IGF-1R). We summarize preclinical studies and the first-in-human study of KW-2450, an oral tyrosine kinase inhibitor with IGF-1R and insulin receptor (IR) inhibitory activity. Preclinical activity of KW-2450 was evaluated in various in vitro and in vivo models. It was then evaluated in a phase I clinical trial in 13 patients with advanced solid tumors (NCT00921336). In vitro, KW-2450 inhibited human IGF-1R and IR kinases (IC50 7.39 and 5.64 nmol/L, respectively) and the growth of various human malignant cell lines. KW-2450 40 mg/kg showed modest growth inhibitory activity and inhibited IGF-1-induced signal transduction in the murine HT-29/GFP colon carcinoma xenograft model. The maximum tolerated dose of KW-2450 was 37.5 mg once daily continuously; dose-limiting toxicity occurred in two of six patients at 50 mg/day (both grade 3 hyperglycemia) and in one of seven patients at 37.5 mg/day (grade 3 rash). Four of 10 evaluable patients showed stable disease. Single-agent KW-2450 was associated with modest antitumor activity in heavily pretreated patients with solid tumors and is being further investigated in combination therapy with lapatinib/letrozole in patients with human epidermal growth factor receptor 2-postive metastatic breast cancer.
The successful application and clinical utility of multitargeted TKIs such as sorafenib1 and sunitinib2 in the treatment of various cancers has accelerated the further discovery and clinical development of multitargeted TKIs.3-6 In vitro profiling of tyrosine kinase inhibition using a panel of kinase enzymes as well as cell-based assays are the most commonly used methods for the evaluation of potential drug candidates. Further understanding of signaling pathway redundancy and cross-talk is also attracting much interest in the evaluation of multitargeted TKIs.7-10 Sorafenib is one of the most representative examples for this paradigm. Sorafenib was designed and discovered as a potent c-Raf kinase inhibitor.1, 11 Subsequent studies revealed that the anticancer activity of sorafenib is also mediated through inhibition of the vascular endothelial growth factor receptor kinase pathway, especially in clinical settings including renal cell carcinoma12 and hepatocellular carcinoma,13 for which sorafenib is now indicated. Furthermore, sorafenib has been found to be a poor inhibitor of B-Raf kinase in patients with melanoma,14 a cancer for which the drug paradoxically shows a degree of clinical activity through inhibition of c-Kit kinase.15 This shows the difficulty in assessing the actual molecular target(s) for multitargeted TKIs towards different cancers.
The IGF family comprises three ligands (IGF-1, IGF-2, and insulin), three corresponding cell membrane receptors (IGF-1R, IGF-2R, and IR), and seven IGF-binding proteins (IGFBP1–7).16 Activation of IGF-1R signaling may contribute to cancer cell proliferation, survival, metastasis, and resistance to anticancer agents in various human malignancies.17-21 Insulin receptor plays an important role in the regulation of IGF function and possible resistance to IGF-1R-targeted therapies, thus suggesting that dual inhibition of IGF-1R and IR may be a viable approach for shutting down the IGF system/axis completely.18, 19, 21
Numerous solid tumors overexpress or have excessively activated IGF-1R22 and several dual IGF-1R/IR TKIs are under clinical investigation.16 KW-2450 is an orally active, indazole-based class of low molecular weight multitargeted TKIs with both IGF-1R and IR inhibitory activity (Fig. 1a). In this report, we summarize preclinical studies of KW-2450 and the first-in-human phase I trial of KW-2450 in patients with solid tumors.
Materials and Methods
Preclinical study methods
Details are provided Data S1.
Clinical trial methods
The primary objective of this study was to establish the safety, tolerability, and RP2D of oral KW-2450 in patients with advanced solid tumors who had not responded to standard therapy or for whom no standard therapy was available. Secondary objectives were to determine the pharmacokinetic profile of KW-2450, to evaluate exploratory pharmacodynamic biomarkers of IGF-1R inhibition by KW-2450 for their potential as markers of therapeutic efficacy, and to evaluate preliminary evidence of efficacy.
This was an open-label, sequential, ascending, multidose, phase I study incorporating a 3+3 dose escalation schedule. Enrolment was to have proceeded until the MTD was established but the first dose was above the MTD, so subsequent doses followed a de-escalation scheme to determine the MTD. The initial dose was determined as 50 mg (approximately 30 mg/m2) per day based on one-sixth of the highest non-severely toxic dose level in dogs (9 mg/kg).
The study was carried out at three institutions in the USA. The protocol, informed consent form, and amendments were reviewed and approved by an institutional review board at each study center. The study was carried out in accordance with the Declaration of Helsinki and International Conference for Harmonization Good Clinical Practice guidelines. All patients provided written informed consent.
Patients with histopathologically or cytologically proven, advanced primary or recurrent solid tumors that had not responded to an adequate course of available therapy, that had progressed or recurred despite an adequate course of available therapy, that was not curable by available therapy, or for which no accepted standard therapy existed were included. Inclusion criteria were: ≥18 years of age; life expectancy >3 months; Eastern Cooperative Oncology Group performance status score ≤2; adequate hematologic (absolute neutrophil count ≥1500/mm3, hemoglobin ≥8.5 g/dL, and platelet count ≥100 000/mm3), hepatic (total bilirubin level ≤1.5 times the ULN, aspartate aminotransferase and alanine aminotransferase ≤2.5 times the ULN or ≤5 times the ULN if known liver metastases), and renal function (serum creatinine ≤1.5 mg/dL for males or ≤1.4 mg/dL for females; or calculated creatinine clearance >60 mL/min based on Cockcroft–Gault formula); and recovered from the effects of any prior antineoplastic therapy. Patients with central nervous system metastases were eligible if they have received prior radiotherapy and/or surgery to site(s) of central nervous system metastatic disease, were off glucocorticoids for ≥4 weeks, were not taking anticonvulsants, and had no overt evidence of neurological deficit. Patients with diabetes, cataract(s), retinopathy, nephropathy, thyroid disease, uncontrolled intercurrent illnesses, unstable cardiovascular disease, HIV, hepatitis B or C, inflammatory diseases of the gastrointestinal tract, malabsorption syndrome, recent major surgery, or any clinically significant deviations in physical examination, vital signs, electrocardiogram, or clinical laboratory determination were excluded. Pharmacological doses of glucocorticoids, strong inhibitors/inducers of CYP3A4/5, herbal medications, drugs for prevention of graft-versus-host disease or transplant rejection, and hematopoietic growth factors or erythropoiesis-stimulating agents were not permitted during and within up to 2 months prior to the administration of study medication. Prior treatment with other agents specifically targeting IGFRs was not permitted. Women could not be pregnant or breast-feeding. All women of child-bearing potential and sexually active men agreed to use effective contraception.
At each dose level, patients received KW-2450 orally once daily for 28 days followed by a 1-week observation period. After completing the 1-week observation period, all patients received KW-2450 on a continuous daily schedule. All doses of KW-2450 were given to patients under overnight fasting conditions.
Response was evaluated every 8 weeks according to Response Evaluation Criteria in Solid Tumors version 1.0 criteria. Adverse events were graded using Common Terminology Criteria for Adverse Events version 3.0 and classed according to relationship to treatment (definite, probable, possible, unlikely, or unrelated). Non-hematologic DLT was defined by: grade 3 fasting hyperglycemia; grade 3 non-fasting hyperglycemia persisting for >4 h; grade 4 hyperglycemia; grade ≥2 cardiac toxicity; and any other grade ≥3 non-hematologic AEs except for symptomatic AEs, such as nausea, vomiting, and diarrhea if they were reduced to grade <3 within 72 h with standard supportive measures. Hematologic DLT was defined by: grade 4 absolute neutrophil count for >7 days; grade 4 thrombocytopenia; and any other toxicity that represented a clinically significant hazard to the patient in the view of the investigator.
Blood samples were collected between days 1 and 29 (24 h after the dose on day 28) for measurement of plasma KW-2450 concentrations, and plasma insulin and glucose. Fasting was continued until 2 h post-dose on these days. Pharmacokinetic analysis was computed by standard non-compartmental methods using WinNonlin version 5.2. The bioanalytical method employed a solid-phase extraction technique to extract KW-2450 from plasma. Extracted plasma samples were quantified using HPLC and tandem mass spectrometry.
Results
Preclinical results
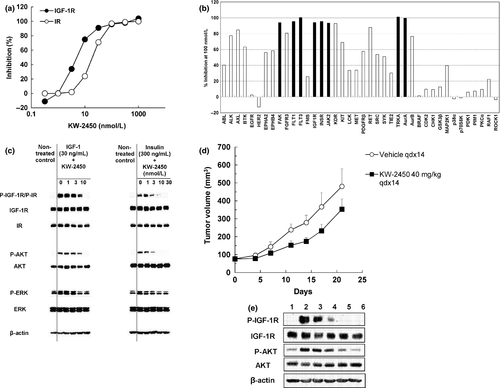
KW-2450 showed high in vitro affinity to IGF-1R and IR tyrosine kinases with IC50 values of 7.39 and 5.64 nmol/L, respectively (Fig. 1a). In addition to IGF-1R and IR kinases, KW-2450 showed potent in vitro inhibitory activity (>90% at 100 nmol/L) against several tyrosine kinases such as focal adhesion kinase, fms-like tyrosine kinase-1 and -3, Jak2, kinase insert domain receptor, tyrosine kinase receptor A, and serine/threonine aurora kinase A (Fig. 1b).
In vitro, KW-2450 inhibited the growth of various types of malignant cells, including cells derived from sarcomas, multiple myelomas, and carcinomas of the colon, pancreas, and lung. Among these, human sarcoma cell lines bearing the EWS/FLI-1 chromosome translocation, such as TC-71, SK-N-MC, and SK-ES-1, were sensitive to KW-2450 with GI50 values of <3.2–11 nmol/L. Human multiple myeloma cell lines, such as KMS-12-BM, LP-1, and NCI-H929, were sensitive to KW-2450 with GI50 values of 11–19 nmol/L. Furthermore, the degree of in vitro inhibition towards the range of human malignant cell lines was highly correlated with that induced by the anti-IGF-1 antibody KM1468 (Fig. S1a).
KW-2450 inhibited IGF-1-induced tyrosine phosphorylation of IGF-1R in the cultured colon cancer cell line HT-29/GFP in a concentration-dependent manner, showing complete inhibition at 30 nmol/L (Fig. 1c). The compound also inhibited insulin-induced tyrosine phosphorylation of IR in the same concentration range, although it showed slightly more potent inhibition than IGF-1R as it showed a significant degree of inhibition at a concentration of 10 nmol/L in the same cells (Fig. 1c). KW-2450 also inhibited phosphorylation of AKT downstream to IGF-1R or IR in the same concentration ranges without any inhibitory activity against phosphorylation of Erk (Fig. 1c).
KW-2450 induced G1 phase accumulation at lower concentrations of 120 and 400 nmol/L (GI50, 380 nmol/L). However, it induced G1 and G2/M phase accumulations at higher concentrations of 1200 and 2000 nmol/L (Fig. S1b).
At 40 mg/kg, KW-2450 showed modest in vivo growth inhibitory activity in the HT-29/GFP colon cancer xenograft model (Fig. 1d). The same dose of KW-2450 inhibited in vivo IGF-1-induced tyrosine phosphorylation of IGF-R in the same model (Fig. 1e).
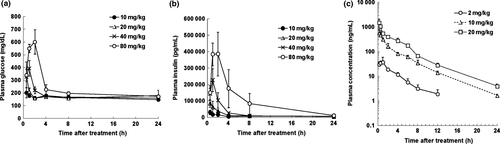
KW-2450 induced elevated levels of plasma glucose (Fig. 2a) and insulin (Fig. 2b) in SCID mice in a dose-dependent manner. These results strongly suggest that it would inhibit the IGF-R/IR axis in animals in vivo. Plasma concentration versus time curves following oral administration of 2, 10, or 20 mg/kg KW-2450 are shown in Figure 2(c). KW-2450 underwent rapid absorption, with a mean time to maximum plasma concentration of 0.25–1 h. Plasma concentrations decreased in a biphasic manner with a mean elimination half-life of 3.71–3.99 h. Mean maximum plasma concentrations were 37.4, 568, and 1510 ng/mL and mean area under the plasma concentration–time curves from time 0 to 24 h were 136, 1320, and 3190 ng/h/mL following 2, 10, and 20 mg/kg KW-2450 doses, respectively. In vitro plasma protein binding analysis at concentrations of 500–10 000 ng Eq/mL KW-2450 revealed the unbound fraction was 4.8–5.7% for murine plasma and 0.9–1.6% for human plasma
Clinical trial results
A total of 13 patients were enrolled and participated in the study from June 2009 to August 2010. All 13 patients received at least one dose of study drug and were included in the safety analysis. Three patients did not have an on-study response assessment, so 10 patients were included in efficacy analysis. Baseline demographic and clinical characteristics are summarized in Table 1 for the intent-to-treat population.
| Characteristics | Total (n = 13) |
|---|---|
| Median age, years (range) | 56 (41–66) |
| Gender, n (%) | |
| Male | 8 (61.5) |
| Female | 5 (38.5) |
| Race | |
| White | 11 (84.6) |
| Black | 1 (7.7) |
| Asian | 1 (7.7) |
| Median height, cm (range) | 169 (151–183) |
| Median weight, kg (range) | 74.4 (50.5–96.4) |
| ECOG performance status, n (%) | |
| 0 | 7 (53.8) |
| 1 | 5 (38.5) |
| 2 | 1 (7.7) |
| Primary tumor site, n (%) | |
| Head/neck | 2 (15.4) |
| Extraskeletal myxoid chondrosarcoma | 1 (7.7) |
| Solitary fibrous sarcoma | 1 (7.7) |
| Melanoma | 1 (7.7) |
| Sweat gland | 1 (7.7) |
| Appendix | 1 (7.7) |
| Breast | 1 (7.7) |
| Fallopian tube | 1 (7.7) |
| Kidney | 1 (7.7) |
| Parotid gland | 1 (7.7) |
| Thymus | 1 (7.7) |
| Rectum | 1 (7.7) |
| Presence of metastatic disease, n (%) | 13 (100) |
| Sites of metastatic disease, n (%) | |
| Lung | 11 (84.6) |
| Liver | 6 (46.2) |
| Bone | 5 (38.5) |
| Lymph nodes | 4 (30.8) |
| Peritoneum | 3 (23.1) |
| Brain | 2 (15.4) |
| Other | 4 (30.8) |
| Stage at study entry, n (%) | |
| IIIB | 2 (15.4) |
| IV | 11 (84.6) |
| Prior therapy, n (%) | |
| Surgery | 13 (100.0) |
| Systemic | 11 (84.6) |
| Radiation | 6 (46.2) |
- ECOG, Eastern Cooperative Oncology Group.
Table 2 summarizes any grade treatment-emergent AEs related to the study drug in more than one patient overall. With respect to DLTs, two of six patients (33.3%) experienced grade 3 non-fasting hyperglycemia at the first dose level of 50 mg/day KW-2450, that is, the dose was above the MTD. One of seven patients (14.3%) had a DLT at the lower dose level of 37.5 mg/day KW-2450; the DLT was grade 3 rash with no patients experiencing grade 3 non-fasting hyperglycemia. The RP2D for KW-2450 was therefore 37.5 mg/day.
| Adverse event | No. of patients (%) | |||||
|---|---|---|---|---|---|---|
| KW-2450 50 mg/day (n = 6) | KW-2450 37.5 mg/day (n = 7) | Total (n = 13) | ||||
| Grade 1–2 | Grade 3 | Grade 1–2 | Grade 3 | Grade 1–2 | Grade 3 | |
| Fatigue | 4 (66.7) | 0 (0.0) | 4 (57.1) | 0 (0.0) | 8 (61.5) | 0 (0.0) |
| Nausea | 4 (66.7) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 6 (46.2) | 0 (0.0) |
| Hyperglycemia | 2 (33.3) | 2 (33.3) | 1 (14.3) | 0 (0.0) | 3 (23.1) | 2 (15.4) |
| Decreased appetite | 3 (50.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (23.1) | 0 (0.0) |
| Dizziness | 2 (33.3) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 3 (23.1) | 0 (0.0) |
| Constipation | 1 (16.7) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 2 (15.4) | 0 (0.0) |
| Diarrhea | 2 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (15.4) | 0 (0.0) |
| Vomiting | 1 (16.7) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 2 (15.4) | 0 (0.0) |
| Mucosal inflammation | 1 (16.7) | 0 (0.0) | 1 (14.3) | 0 (0.0) | 2 (15.4) | 0 (0.0) |
| Pain | 2 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (15.4) | 0 (0.0) |
| Anorexia | 0 (0.0) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 2 (15.4) | 0 (0.0) |
| Rash | 0 (0.0) | 0 (0.0) | 1 (14.3) | 1 (14.3) | 1 (7.1) | 1 (7.1) |
- Adverse events were graded using Common Terminology Criteria for Adverse Events version 3.0. For the three patients who had treatment-related grade 3 adverse events (hyperglycemia in two patients on 50 mg/day and rash in one patient on 37.5 mg/day), each was classed as a dose-limiting toxicity. There were no other treatment-related grade 3 adverse events. There were no grade 4 adverse events (regardless of relationship to treatment).
Table 3 summarizes pharmacokinetic variables for KW-2450 following the first oral dose and after 28 days of continuous treatment with 37.5 or 50 mg KW-2450 once daily. KW-2450 was rapidly absorbed with mean maximum plasma concentrations ranging from 1.50 to 2.29 h across dose levels and day measured. Mean elimination half-life ranged from 9.74 to 13.0 h across dose levels and day measured. Mean maximum plasma KW-2450 concentration was 2329 and 2366 ng/mL on days 1 and 28, respectively, for the 37.5 mg dose, and 3030 and 2970 ng/mL, respectively, for the 50 mg dose. Mean trough plasma KW-2450 concentration was 608 and 890 ng/mL on days 1 and 28, respectively, for the 37.5 mg dose, and 671 and 701 ng/mL, respectively, for the 50 mg dose. There was slight accumulation on multiple oral dosing of KW-2450 as the mean accumulation ratio on day 28 was 1.57 and 1.27 for the 37.5 and 50 mg doses, respectively.
| Pharmacokinetic parameter | Mean ± SD | |||
|---|---|---|---|---|
| KW-2450 37.5 mg/day | KW-2450 50 mg/day | |||
| Day 1 (n = 7) | Day 28 (n = 5) | Day 1 (n = 6) | Day 28 (n = 4) | |
| Tmax (h) | 2.29 ± 0.95 | 1.99 ± 1.22 | 1.86 ± 0.73 | 1.50 ± 0.58 |
| Cmax (ng/mL) | 2329 ± 921 | 2366 ± 1014 | 3030 ± 1070 | 2970 ± 373 |
| Cmin (ng/mL) | 608 ± 679 | 890 ± 1173 | 671 ± 517 | 701 ± 462 |
| AUC0–24 (ng/h/mL) | 28 233 ± 19 543 | 34 420 ± 26 340 | 35 224 ± 15 742 | 31 973 ± 11 613 |
| t1/2 (h) | 10.1 ± 3.08† | 9.74 ± 2.69‡ | 11.0 ± 4.16† | 13.0 ± 4.18 |
| CL/F (L/h) | 1.45 ± 0.53† | 1.56 ± 0.88 | 1.48 ± 0.97† | 1.69 ± 0.47 |
| VZ/F (L) | 19.6 ± 2.91† | 23.7 ± 3.89‡ | 19.7 ± 8.32† | 29.6 ± 1.69 |
| R | NA | 1.57 ± 0.80 | NA | 1.27 ± 0.37 |
- †n = 5; ‡n = 4. AUC0–24, area under the plasma concentration–time curve from 0 to 24 h; Cmax, maximum plasma concentration; Cmin, trough plasma concentration; CL/F, apparent clearance; NA, not applicable; R, accumulation ratio (AUC0–24 on day 28/AUC0–24 on day 1); t1/2, elimination half-life; Tmax, time to Cmax; VZ/F, apparent volume of distribution.
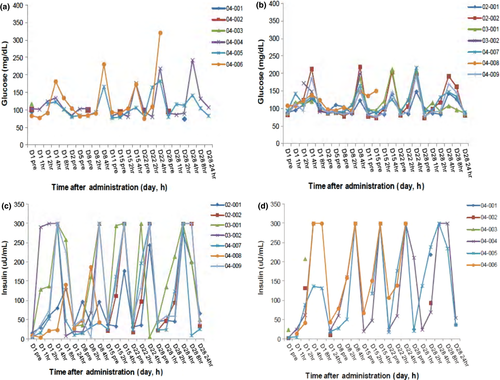
On the days requiring pharmacodynamic sampling (and pharmacokinetic sampling), the daily dose of KW-2450 was given after overnight fasting and a 2-h fast was required post-dose. The effect of KW-2450 alone can therefore only be assessed at the fasting time points, that is, pre-dose, and at 1 and 2 h post-dose. Elevations in fasting glucose (Fig. 3a,b) and insulin (Fig. 3c,d) were noted at 1 and 2 h post-dose. Mean pre-dose glucose values remained within the normal range and mean glucose values returned to pre-dose levels by 24 h post-dose on days 1 and 29 (data not shown). Insulin values approached baseline levels by 24 h post-dose. Insulin values above the upper limit of quantitation (300 μU/mL) were noted in five patients in the 37.5-mg cohort and three patients in the 50-mg cohort. These samples were almost entirely collected from patients in a non-fasting state at 4- and 8-h time points, although some were at 2-h time points. Increases in fasting mean insulin values were observed from pre-dose to 2 h post-dose in each dose cohort.
For the 10 patients evaluable for response, there were no complete or partial responses, four patients (40%) had stable disease (50 mg/day, n = 3; 37.5 mg/day, n = 1), and the remaining patients had progressive disease. Median progression-free survival was 141 days (95% CI, 50.0–414 days) at 50 mg/day, 65.0 days (95% CI, 40.0–67.0 days) at 37.5 mg/day, and 66.5 days (95% CI, 50.0–112.0 days) overall.
Discussion
This was the first-in-human clinical study of KW-2450. The MTD and RP2D was 37.5 mg once daily given orally on a continuous basis. Two of six patients in the 50-mg/day cohort experienced grade 3 hyperglycemia as a DLT. The only DLT in the 37.5-mg/day cohort was grade 3 rash in one of seven patients. The pharmacokinetic study indicated that KW-2450 was rapidly absorbed following oral administration with a mean elimination half-life of approximately 10–13 h. KW-2450 accumulated slightly on once-daily dosing over the first 4 weeks of treatment, by the end of which time steady state should have been attained. Mean maximum plasma KW-2450 concentration was 2329 and 2366 ng/mL on days 1 and 28, respectively, for the MTD dose of 37.5 mg/day. This is equivalent to 3385–3440 nmol/L. As KW-2450 is extensively protein bound (98.5–99.0%) (Kyowa Hakko Kirin Co., Ltd, Tokyo, unpublished data), the unbound concentration of KW-2450 is estimated to be ~50 ng/mL (~75 nmol/L). This concentration is above the IC50 values for in vitro inhibition of IGF-1R and IR (7.39 and 5.64 nmol/L, respectively). This indicates that the MTD of 37.5 mg provides KW-2450 concentrations above the level needed for pharmacologic activity. Evidence of robust pharmacologic activity was also observed in relation to the increase in fasting plasma insulin and glucose levels in both dose groups.
The initial in vitro profiling of tyrosine kinase inhibition by KW-2450, shown in Figure 1(b), suggested that KW-2450 would be a multitargeted TKI. However, the phase I clinical study showed that KW-2450 acted as a selective IGF-1R/IR TKI in humans at doses below the MTD. This might also have been suggested from non-human primate toxicological studies as well as rodent in vivo studies, which showed hyperglycemia induced at pharmacologically active (lower) doses (Kyowa Hakko Kirin Co., Tokyo, unpublished data). Table 4 summarizes the in vitro profiles of tyrosine kinase inhibition for KW-2450 compared to other IGF-1R/IR dual kinase inhibitors, linsitinib (OSI-906),23 and BMS-754807.24 The degree of in vitro inhibition toward a range of human malignant cell lines at the IC50 was highly correlated with that induced by the anti-IGF-1 antibody, KM1468 (Fig. 1c). Additionally, KW-2450 induced G2M arrest (Fig. 1c), which could be mediated by the possible inhibition of aurora kinases;25 this paradigm is evident at higher concentrations in vitro and higher doses in vivo. Overall, these results show the difficulty in assessing the actual molecular target(s) of multitargeted TKIs.
| Kinase | KW-2450 | BMS-754807 | Linsitinib | |
|---|---|---|---|---|
| IC50, nmol/L | Inhibition at 100 nmol/L, % | IC50, nmol/L | IC50, nmol/L | |
| IGF-1R | 7.39 | 94.1 | 1.8 | 35 |
| IR | 5.64 | 95.6 | 1.7 | 75 |
| TrkA | NT | 101.1 | 7.4 | >10 000 |
| Jak2 | NT | 93.4 | 347.0 | >10 000 |
| AurA | NT | 99.5 | 9.0 | >10 000 |
| AurB | NT | 76.8 | 25.0 | >10 000 |
| Btc | NT | 63.2 | 3797.0 | >10 000 |
| Src | NT | 53.8 | 1527.0 | >10 000 |
| Lck | NT | 33.7 | 1218.0 | >10 000 |
| Met | NT | 33.8 | 5.6 | >10 000 |
| EGFR | NT | 2.5 | >50 000 | >10 000 |
| p381 | NT | –2.5 | >50 000 | >10 000 |
- Data for KW-2450 derived from the present report and those for linsitinib23 and BMS-75480724 from previously published reports. AurA, aurora kinase A; AurB, aurora kinase B; Btc, betacellulin; EGFR, epidermal growth factor receptor; IGF-1R, insulin-like growth factor receptor-1; IR, insulin receptor; Lck, lymphocyte-specific protein tyrosine kinase; NT, not tested; TrkA, Tropomyosin-receptor kinase A.
As a single agent, treatment with KW-2450 was associated with a best response of stable disease in this heavily pretreated patient population, with four of 10 evaluable patients showing stable disease. Early clinical studies for other IGF-1R/IR dual kinase inhibitors such as linsitinib and BMS-754807 have also suggested only modest activity as monotherapy in early clinical trials,26-28 although combination therapy with other agents has indicated more favorable response rates with paclitaxel and erlotinib29, 30 but not with everolimus.31 In a recently published phase III study,32 linsitinib as monotherapy did not increase overall or progression-free survival compared to placebo in patients with metastatic adrenocortical carcinoma. Given the limited number of patients treated with KW-2450 in our study, it is not possible to make a meaningful comparison of efficacy with other IGF-1R/IR dual kinases inhibitors, linsitinib and BMS-754807, given as single-agent therapy, although all three agents showed severe hyperglycemia as the most frequent DLT and/or most common treatment-related AE, suggesting a common safety profile indicative of IGF-1R/IR kinase inhibition.
Preliminary in vitro and in vivo studies have indicated that KW-2450 exerts synergistic antitumor effects in combination with lapatinib, an epidermal growth factor receptor/HER2 kinase inhibitor approved for treatment of HER2-positive metastatic breast cancer, and letrozole, an aromatase inhibitor approved for estrogen-positive metastatic breast cancer (Kyowa Hakko Kirin Co., Tokyo, unpublished data). A phase I/II clinical trial is underway to evaluate the safety, tolerability, and preliminary efficacy of KW-2450 in combination with lapatinib plus letrozole in patients with HER2-positive advanced or metastatic breast cancer.
Acknowledgments
The authors thank Peter Todd (Tajut Ltd, Kaiapoi, New Zealand) for medical editorial assistance with this article, for which he received financial compensation from Kyowa Hakko Kirin Pharma, Inc. (Princeton, NJ, USA).
Disclosure Statement
Y.M., Y.W., N.K., D.N., and S.A. are employees of Kyowa Hakko Kirin. The other authors have no conflict of interest. The studies were designed under the responsibility of Kyowa Hakko Kirin, in conjunction with the steering committee for the clinical study. Kyowa Hakko Kirin collected and analyzed the data and contributed to the interpretation of the study.
Abbreviations
-
- AE
-
- adverse event
-
- CI
-
- confidence interval
-
- DLT
-
- dose-limiting toxicity
-
- GI50
-
- 50% growth inhibitory concentration
-
- HER2
-
- human epidermal growth factor receptor 2
-
- IGF-1R
-
- insulin-like growth factor receptor-1
-
- IGF
-
- insulin-like growth factor
-
- IR
-
- insulin receptor
-
- MTD
-
- maximum tolerated dose
-
- RP2D
-
- recommended phase II dose
-
- TKI
-
- tyrosine kinase inhibitor
-
- ULN
-
- upper limit of normal




