Anti-bevacizumab idiotype antibody vaccination is effective in inducing vascular endothelial growth factor-binding response, impairing tumor outgrowth
Funding Information:
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Fundação de Apoio à Pesquisa do Estado de São Paulo.
Abstract
Tumors require blood supply and, to overcome this restriction, induce angiogenesis. Vascular endothelial growth factor (VEGF) plays an important role in this process, which explains the great number of antiangiogenic therapies targeting VEGF. The research and development of targeted therapy has led to the approval of bevacizumab, a humanized anti-VEGF monoclonal antibody (mAb), in clinical settings. However, side effects have been reported, usually as a consequence of bolus-dose administration of the antibody. This limitation could be circumvented through the use of anti-idiotype (Id) antibodies. In the present study, we evaluated the efficacy of an active VEGF-binding immune response generated by an anti-bevacizumab idiotype mAb, 10.D7. The 10.D7 anti-Id mAb vaccination led to detectable levels of VEGF-binding anti-anti-Id antibodies. In order to examine whether this humoral immune response could have implications for tumor development, 10.D7-immunized mice were challenged with B16-F10 tumor cells. Mice immunized with 10.D7 anti-Id mAb revealed reduced tumor growth when compared to control groups. Histological analyses of tumor sections from 10.D7-immunized mice showed increased necrotic areas, decreased CD31-positive vascular density and reduced CD68-positive cell infiltration. Our results encourage further therapeutic studies, particularly if one considers that the anti-Id therapeutic vaccination maintains stable levels of VEGF-binding antibodies, which might be useful in the control of tumor relapse.
Like any tissue, tumors require blood supply.1 The vascular endothelial growth factor (VEGF) is a key angiogenic factor secreted by several tumors, at least in their initial stages.2 Thus, therapies targeting angiogenesis have a place in treating human cancer. A humanized anti-VEGF monoclonal antibody (mAb), bevacizumab, has been approved for use, with other drugs, for certain tumors.3, 4 However, clinical studies show that the use of bevacizumab is associated with some side effects, such as gastrointestinal bleeding, thrombotic events, epistaxis and hypertensive episodes.3, 5 These complications are related to the weekly or the 2-week administrations of antibody in massive doses, which often prevent continuing treatment.3
Taking into account our experience with anti-idiotype (anti-Id) antibodies,6, 7 we considered that the idiotype network approach could circumvent the need for administration of high doses of anti-VEGF antibodies. In contrast to this, therapeutic vaccination with an anti-Id mAb stimulates an active immune response, usually of low but sustained intensity.
Therefore, the aim of the present study was to prove the concept that it is possible to obtain a VEGF mimic anti-Id antibody. For this, we examined whether the anti-Id mAb immunization triggers a VEGF-binding antibody response able to inhibit angiogenesis and, thereby, interfere with the tumor growth.
Materials and Methods
Hybridoma, HUVEC and B16-F10 melanoma cells were cultured as described previously.8 Antibody generation was obtained by hybridoma technology6 and VEGF-binding antibody detection by ELISA.9 Detailed procedures are described in Data S1.
Results
Generation of anti-bevacizumab idiotype monoclonal antibody, 10.D7
To generate an anti-bevacizumab idiotype antibody, four BALB/C mice were immunized with KLH-conjugated bevacizumab. Screening was based on the inhibition of biotinylated bevacizumab binding to hVEGF in the presence of immune serum. After limiting dilution cloning, stable anti-bevacizumab idiotype-secreting hybridoma clone 10.D7 was selected and the secreted antibody was determined to be an IgG1 isotype.
Therapeutic vaccination with 10.D7 anti-idiotype monoclonal antibody impairs tumor growth and angiogenesis
We used the B16-10 tumor model to examine the therapeutic potential of our proposed anti-Id mAb vaccination. Mice were challenged with B16-F10 cells on the 10th day after boost immunization, according to the experimental schedule shown in Figure 1a. Vaccination with 10.D7 anti-Id mAb resulted in significantly reduced tumor growth, compared to the adjuvant and isotype immunized control groups (P < 0.05; one-way anova). Tumor growth curves are plotted in Figure 1b.
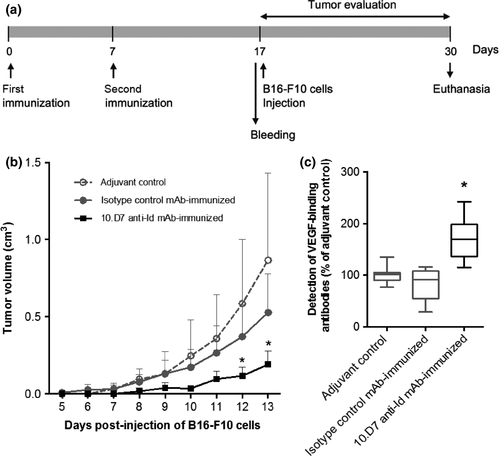
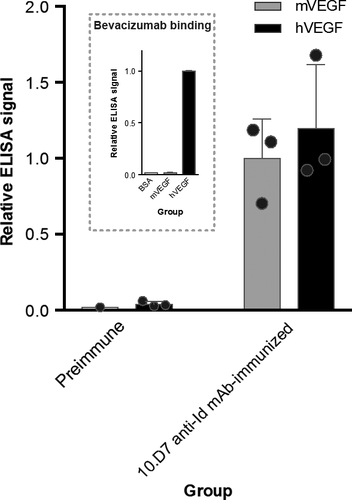
The presence of detectable levels of hVEGF-binding antibodies in serum samples from 10.D7 anti-Id mAb-immunized mice immediately before B16-F10 cell injection was verified by ELISA (Fig. 1c). It is important to observe here that such immune sera recognize not only hVEGF but also the murine form of this factor. Bevacizumab, in comparison, could bind only to hVEGF, as expected (Fig. 2).
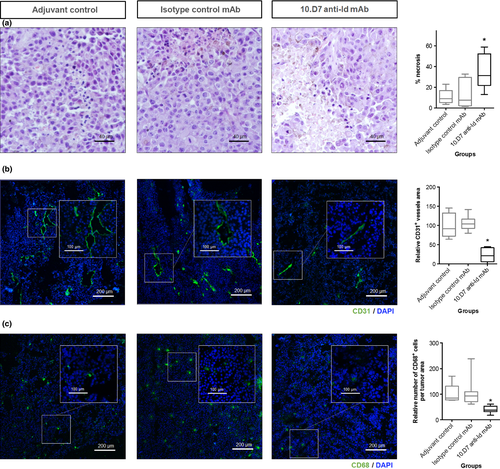
Histological analyses of HE-stained sections revealed an increase of necrotic area in tumors from mice immunized with 10.D7 anti-Id mAb: the mean percentage of tumor necrotic area was approximately fivefold higher than that observed in the control groups (P < 0.05; one-way anova). Representative HE-stained sections and the respective necrosis quantification are shown in Figure 3a. This result was accompanied by a decreased CD31-positive vascular network in subcutaneous tumors excised from the 10.D7 anti-Id mAb-immunized group, compared to controls (P < 0.05; one-way anova; Fig. 3b). In addition, a reduced number of CD68-positive cells were observed in tumors from 10.D7 mAb-immunized mice (P < 0.05, one-way anova) (Fig. 3c).
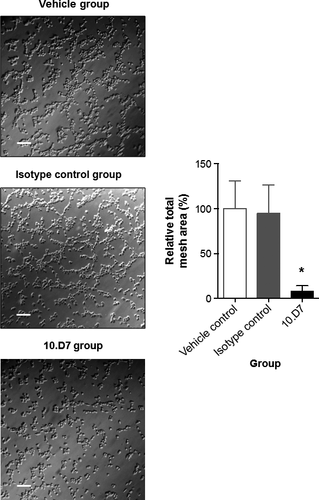
Furthermore, such angiogenic inhibition was also observed by in vitro tubulogenesis assay. As shown in Figure 4, HUVEC incubation with sera obtained after 10.D7 anti-Id mAb immunization gave a reduced and poorly organized capillary-like plexus, compared with the serum controls (P < 0.05; one-way anova).
Discussion
The use of anti-Id antibodies acting as an internal image of tumor-expressing antigens on vaccination settings offers the opportunity to overcome tolerance to auto-antigens10 and to control tumor progression,11, 12 with some studies already in clinical development (Table S1). Herein, we show the effectiveness of an anti-bevacizumab idiotype antibody in generating an active VEGF-binding immune response. As far as we know, this is the first time that an anti-Id approach has been used to inhibit angiogenesis.
The B16-F10 tumor model was chosen to investigate the therapeutic viability of our anti-bevacizumab idiotype mAb vaccination approach. Despite clinical studies pointing to the non-responsiveness of melanomas to VEGF-targeting therapies,13, 14 the previous reported impairments of B16-F10 solid tumor growth and vascular density by anti-VEGF antibodies15, 16 provide a rationale for the use of this experimental mouse model.
The effectiveness of the studied anti-Id mAb vaccination was revealed by the detection of serum VEGF-binding antibodies, in B16-F10-challenged animals, leading to tumors with reduced size and higher necrotic areas, compared to those obtained in the control groups. The antiangiogenic activity obtained by anti-Id vaccination was evidenced by the significantly lower CD31-positive tumor vascular area, as well as by the hampered in vitro formation of tube-like structures of HUVEC, known to secrete VEGF.17 These results are in agreement with previous reports stating that VEGF inhibition, mainly in the early stages of tumor growth, impairs tumor angiogenesis and its further progression.16, 18
It is already known that even though bevacizumab seems to interact with degraded products of murine VEGF,19 it fails in inhibiting mVEGF-induced activity and in displaying any effect on murine tumor growth.20 Despite this, sera from mice immunized with 10.D7 anti-bevacizumab idiotype antibody recognize both murine and human VEGF, which makes it reasonable to assume that the in vivo data presented here might be, at least in part, the result of mVEGF immunoneutralization by VEGF-binding antibody serum content.
Given that VEGF acts in several processes in the tumor microenvironment, mechanisms other than impairing tumor angiogenesis might be behind our therapeutic findings. Of note, it is known that VEGF blocking strategies led to reduced recruitment and retention of circulating myeloid cells, like monocytes, to the perivascular area,21 as well as to enhanced activation of dendritic cells.22 In fact, analyses of tumors from 10.D7 mAb-immunized mice pointed in the same direction. In addition, Fc-mediated mechanisms, such as complement- or antibody dependent cell-mediated cytotoxicities, might contribute to the reduced tumor growth observed.
Overall, mice immunization with an anti-bevacizumab idiotype antibody was effective in eliciting VEGF-binding humoral IgG antibodies capable of impairing vascular density and tumor growth. The proposed vaccination should be considered as an antiangiogenic therapeutic strategy for controlling the progression of VEGF-dependent tumors, including post-surgical resection relapse scenarios, where VEGF plays an important role.23, 24
Acknowledgments
This work was supported by FAPESP (98/14247-6; 09/18631-1; 12/24280-0) and CAPES (JSS; CBP; RBA) research grants.
Disclosure Statement
The authors have no conflict of interest to declare.




