Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2 and Toll-like receptor/MyD88 pathways
Funding Information:
Japan Agency for Medical Research and Development, (Grant/Award Number: ‘AMED-CREST (Chronic Inflammation)’) Ministry of Education, Culture, Sports, Science, and Technology, (Grant/Award Number: ‘Grants-in-Aids for Young Scientists (15K18405)’)
Abstract
Cyclooxygenase-2 (COX-2) and its downstream product prostaglandin E2 (PGE2) play a key role in generation of the inflammatory microenvironment in tumor tissues. Gastric cancer is closely associated with Helicobacter pylori infection, which stimulates innate immune responses through Toll-like receptors (TLRs), inducing COX-2/PGE2 pathway through nuclear factor-κB activation. A pathway analysis of human gastric cancer shows that both the COX-2 pathway and Wnt/β-catenin signaling are significantly activated in tubular-type gastric cancer, and basal levels of these pathways are also increased in other types of gastric cancer. Expression of interleukin-11, chemokine (C-X-C motif) ligand 1 (CXCL1), CXCL2, and CXCL5, which play tumor-promoting roles through a variety of mechanisms, is induced in a COX-2/PGE2 pathway-dependent manner in both human and mouse gastric tumors. Moreover, the COX-2/PGE2 pathway plays an important role in the maintenance of stemness with expression of stem cell markers, including CD44, Prom1, and Sox9, which are induced in both gastritis and gastric tumors through a COX-2/PGE2-dependent mechanism. In contrast, disruption of Myd88 results in suppression of the inflammatory microenvironment in gastric tumors even when the COX-2/PGE2 pathway is activated, indicating that the interplay of the COX-2/PGE2 and TLR/MyD88 pathways is needed for inflammatory response in tumor tissues. Furthermore, TLR2/MyD88 signaling plays a role in maintenance of stemness in normal stem cells as well as gastric tumor cells. Accordingly, these results suggest that targeting the COX-2/PGE2 pathway together with TLR/MyD88 signaling, which would suppress the inflammatory microenvironment and maintenance of stemness, could be an effective preventive or therapeutic strategy for gastric cancer.
Epidemiological studies have indicated that regular use of non-steroidal anti-inflammatory drugs is associated with a reduced risk of gastrointestinal cancer, suggesting that inflammation plays a role in cancer development.1, 2 Target molecules of non-steroidal anti-inflammatory drugs are COX-1 and COX-2, which catalyze prostaglandin biosynthesis. Genetic studies have indicated that COX-2 and its downstream product PGE2 play a key role in gastrointestinal tumorigenesis.3-5 In cancer tissues, macrophages infiltrate and are activated with the induction of the COX-2/PGE2 pathway, which further induces cytokine signaling.6, 7 The transcription factors NF-κB and Stat3 are activated by inflammatory cytokines, which plays an important role in tumorigenesis.8-10 Based on these results, it has been established that inflammation promotes cancer development through induction of the COX-2/PGE2 pathway and activation of NF-κB and Stat3.11-13 Innate immune responses through the TLRs have also been shown to be involved in tumor promotion.14 We have shown that the COX-2/PGE2 pathway triggers generation of inflammatory microenvironment in the stomach in a mouse gastric tumor model,5, 15 and that TLR signaling through the adaptor molecule MyD88 is required for COX-2/PGE2-induced gastritis and gastric tumor development.16 Accordingly, it is possible that the interplay of the COX-2/PGE2 and TLR/MyD88 pathways is important for generation of the inflammatory microenvironment, which promotes tumorigenesis.
In this review, we define the phenotypes of our gastric tumor model (Gan mice that develop COX-2/PGE2 pathway-dependent gastric tumors) by comparing them to human gastric cancers and discuss the role of COX-2/PGE2-associated inflammation in gastric tumorigenesis and its connection to the TLR/MyD88 pathways.
Gan mice recapitulate human tubular-type gastric cancer
Cyclooxygenase-2 expression is induced in most cancers, and the role of the COX-2/PGE2 pathway was first examined in intestinal tumors.12 Using ApcΔ716 mice, a model of familial adenomatous polyposis, we have shown that COX-2/PGE2 signaling is required for intestinal tumorigenesis and that treatment with a COX-2 inhibitor significantly suppressed intestinal tumorigenesis.3, 17-19 Thus far, a number of mechanisms of PGE2 signaling have been reported to be involved in tumor promotion, including angiogenesis,20 tumor cell survival,21 silencing of tumor suppressors by DNA methylation,22 and recruitment of MDSCs.23
Induction of COX-2 expression is found in more than 90% of gastric cancers,24 and Helicobacter pylori infection is known to be a major cause of COX-2 induction.25 To examine the role of the COX-2/PGE2 pathway in the stomach, we constructed K19-C2mE mice that express Ptgs2 and Ptges, encoding COX-2 and microsomal prostaglandin E synthase-1, respectively, using the Krt19 gene promoter (Data S1). The PGE2 level is significantly increased in the gastric mucosa of K19-C2mE mice, which develop gastritis with macrophage infiltration, indicating that the COX-2/PGE2 pathway triggers inflammatory response.15, 26
Inflammation promotes tumorigenesis in cooperation with oncogenic activation, and accumulation of β-catenin, a hallmark of Wnt signaling activation, is found in more than 50% of gastric cancers,5 although genetic alterations in APC and the β-catenin gene (CTNNB1) were found only in 24/223 (10.8%) and 6/223 (2.7%) of gastric cancers, respectively.27 It is possible that Wnt signaling is activated in gastric cancer also by mechanisms other than APC or CTNNB1 mutations. We thus constructed K19-Wnt1 transgenic mice that express Wnt1, a ligand for canonical Wnt signaling, in gastric epithelial cells. Notably, K19-Wnt1 mice develop small dysplastic lesions in the gastric mucosa; however, simultaneous activation of Wnt signaling and the COX-2/PGE2 pathway in compound K19-Wnt1 and K19-C2mE mice (Gan mice) results in the development of inflammation-associated gastric tumors.5, 15 Histologically, Gan mouse tumors consist of a glandular structure with irregular branching, and are similar to that of human tubular-type gastric cancer (Fig. 1a, Data S1). In Gan mouse tumors, F4/80 positive macrophages and a small number of lymphocytes infiltrate the tumor stroma. Accordingly, it is possible that the COX-2/PGE2 pathway induces the inflammatory response, but is not sufficient to induce acquired immune responses. Notably, depletion of macrophages in Gan mouse tumors caused apoptosis of tumor cells, indicating a role of macrophages in tumor cell survival.28
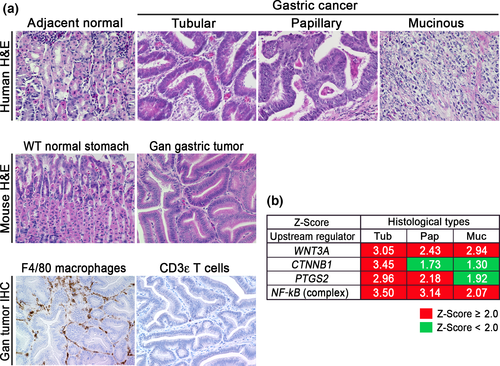
The results of a comprehensive molecular evaluation of human gastric cancers have been reported in the TCGA project.29 Moreover, the Asian Cancer Research Group developed a novel classification of clinically relevant molecular subtypes of gastric cancer.27 In the present study, we examined the signaling pathways that are activated in the human gastric cancer subtypes using the TCGA database. We extracted expression data from the respective histological types (tubular-type, papillary-type, and mucinous-type gastric cancer) and carried out an Ingenuity pathway analysis (Fig. 1b). Notably, both of the canonical Wnt signaling pathways (WNT3 and CTNNB1) were significantly activated in tubular-type gastric cancer. In contrast, NF-κB activation was found in all types of gastric cancer, indicating inflammatory responses in all gastric cancer types. Moreover, the PTGS2 pathway was significantly activated in tubular-type and papillary-type gastric cancer. Accordingly, Wnt signaling and the COX-2/PGE2 pathways are simultaneously activated in tubular-type gastric cancer, although the basal levels of these pathways are increased in all types compared with normal stomach.
Based on the histological characteristics and expression profiles, we concluded that Gan mice recapitulate human tubular-type gastric cancer. That is, the combined activation of Wnt signaling and the COX-2/PGE2 pathway can cause tubular-type gastric cancer. This finding is consistent with our previous microarray analyses, showing that expression profiles of Gan mouse tumors are similar to intestinal-type gastric cancer (Lauren's classification).30
Roles of COX-2/PGE2 pathway-associated inflammation in stemness
Recently, PGE2 has been revealed to be involved in the maintenance of stemness. Treatment of irradiated mice with PGE2 results in increased survival of hematopoietic stem cells,31 and PGE2 signaling regulates hematopoietic stem cell self-renewal through direct interaction with Wnt signaling by cAMP/protein kinase A activation.32, 33 The PGE2/Wnt signaling interaction also regulates regeneration of other organs including liver.32 More recently, it has been shown that PGE2 signaling through the EP4 receptor induces expansion of CD133+ CD44+ cancer stem cell populations in intestinal tumors through the activation of phosphatidylinositol 3-kinase and MAPK signaling.34
We therefore examined the COX-2/PGE2 pathway-dependent upregulated genes in mouse gastritis and tumors using the RNA sequencing results (GSE70135) (Data S1).16 A principal component analysis indicated the similarity of the expression profile of Gan mouse tumors to that of K19-C2mE gastritis (Fig. 2a), in both of which COX-2/PGE2 pathway is induced. A clustering analysis revealed that the genes encoding stem cell-related factors including CD44, Sox2, Noxo1, Cd14, Ephb2, Cxcr4, Sox9, and Prom1, are upregulated in both gastritis and gastric tumors compared to normal stomach (Fig. 2b,c). These genes are also upregulated in Lgr5+VE normal gastric stem cells.35 Moreover, it has been shown recently that Cxcr4-expressing innate lymphoid cells construct perivascular niche for Mist1-expressing gastric stem cells.36 On the other hand, Kcne2, Trim50, and Hrh2 were significantly downregulated in both gastritis and gastric tumors. These molecules are involved in acid secretion from differentiated epithelia. Accordingly, it is possible that the COX-2/PGE2 pathway promotes gastric tumorigenesis through maintenance of stemness by cell-intrinsic mechanisms or by stem cell niche construction.
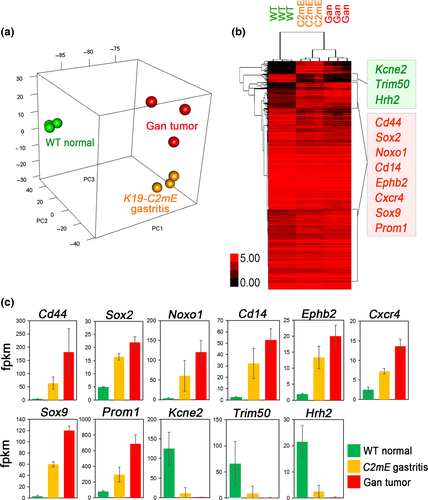
It has been shown that TNF-α, IL-6, and CXCL12 signals activate each other, inducing the cytokine network in tumor tissues.37 Accordingly, inhibition of TNF-α may disturb the cytokine network in tumor microenvironment. Importantly, disruption of the TNF-α gene (Tnf) in BMDCs of Gan mice significantly suppressed gastric tumorigenesis, and expression levels of stem cell markers (CD44, Prom1, Sox9, and Noxo1) were significantly downregulated.38 Accordingly, it is possible that the TNF-α cytokine network, which is activated by the COX-2/PGE2 pathway, plays an important role in maintenance of tumor cell stemness.
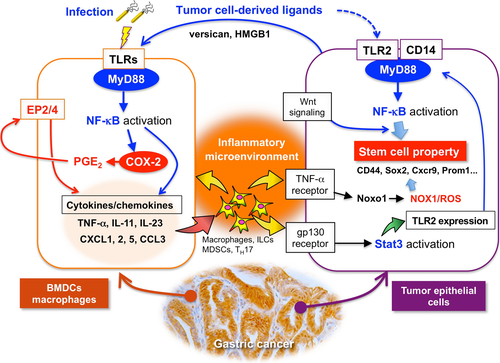
Among the TNF-α-dependent upregulated genes in tumor cells, we noticed that Noxo1 expression is required for the soft-agar colony formation of gastric cancer cells.38 Noxo1 is a component of the NOX1 complex, and plays a role in ROS production.39 NOX1 is involved in tumor promotion through inactivation of phosphatase and tensin homolog and SHP2, and ablation of NOX1 expression resulted in growth arrest of Ras-transformed cells.39-41 Moreover, it has been shown that NOX1/ROS signaling activates NF-κB, which contributes to maintenance of stemness in intestinal tumor cells.42 Accordingly, it is possible that the NOX1/ROS pathway is one of the key mechanisms that link the COX-2/PGE2-dependent inflammatory microenvironment and cancer stem cell regulation (Fig. 3).
However, it has been shown that the mechanism by which a variant-form CD44 protects against ROS is important for gastric tumorigenesis.43 Accordingly, it is conceivable that high ROS levels are detrimental to tumor cells and that the antioxidant mechanism is important for tumorigenesis, while ROS signaling may also promote early-stage tumorigenesis.44
“Infection-associated” and “tumor-elicited” inflammation
Approximately 20% of malignant cancers are associated with infection.45, 46 Gastric cancer, liver cancer, and cervical cancer are associated with H. pylori, hepatitis C virus, and human papilloma virus infection, respectively, and are included in this group. Helicobacter pylori promotes gastric cancer by injecting CagA into host cells, which binds cellular SHP2 or partitioning-defective 1, resulting in disturbance of cell function.47 At the same time, H. pylori stimulates TLRs, leading to activation of innate immunity to induce “infection-associated inflammation” and generate the inflammatory microenvironment.48
Chronic inflammation, conversely, is associated with most cancers that are not related to infection. In cancer tissues, tumor cell-derived factors can also stimulate TLRs as damage-associated molecular patterns to induce “tumor-elicited inflammation”.49, 50
We therefore compared the expression profiles of cytokines and chemokines in human gastric cancer (TCGA database) with those of H. pylori infection-associated human gastritis (GSE60662) (Table 1).51 Interestingly, expression patterns of inflammation-related factors were not always consistent in gastritis and gastric cancer. For example, expression of IL-8 and IL-11 is predominantly induced in gastric cancer, whereas TNF-α expression is mostly increased in gastritis. It is possible that “tumor-elicited inflammation” is also induced in gastric cancer, which may result in cytokine profiles that differ from H. pylori infection-induced gastritis. However, CXCL1, CXCL2, CXCL5, CCL3, CCL4, and TLR2 are upregulated in both gastritis and gastric cancer (Table 1). Notably, these genes are induced also in both K19-C2mE gastritis and Gan gastric tumors. It is therefore possible that these inflammatory factors are induced in a COX-2/PGE2 pathway-dependent manner, regardless of the mechanisms that activate TLRs. Although IL-11 is not significantly induced in human gastritis, it is upregulated in mouse gastritis, suggesting that it is induced through the COX-2/PGE2 pathway. Importantly, these listed cytokines and chemokines have tumor-promoting functions as follows.
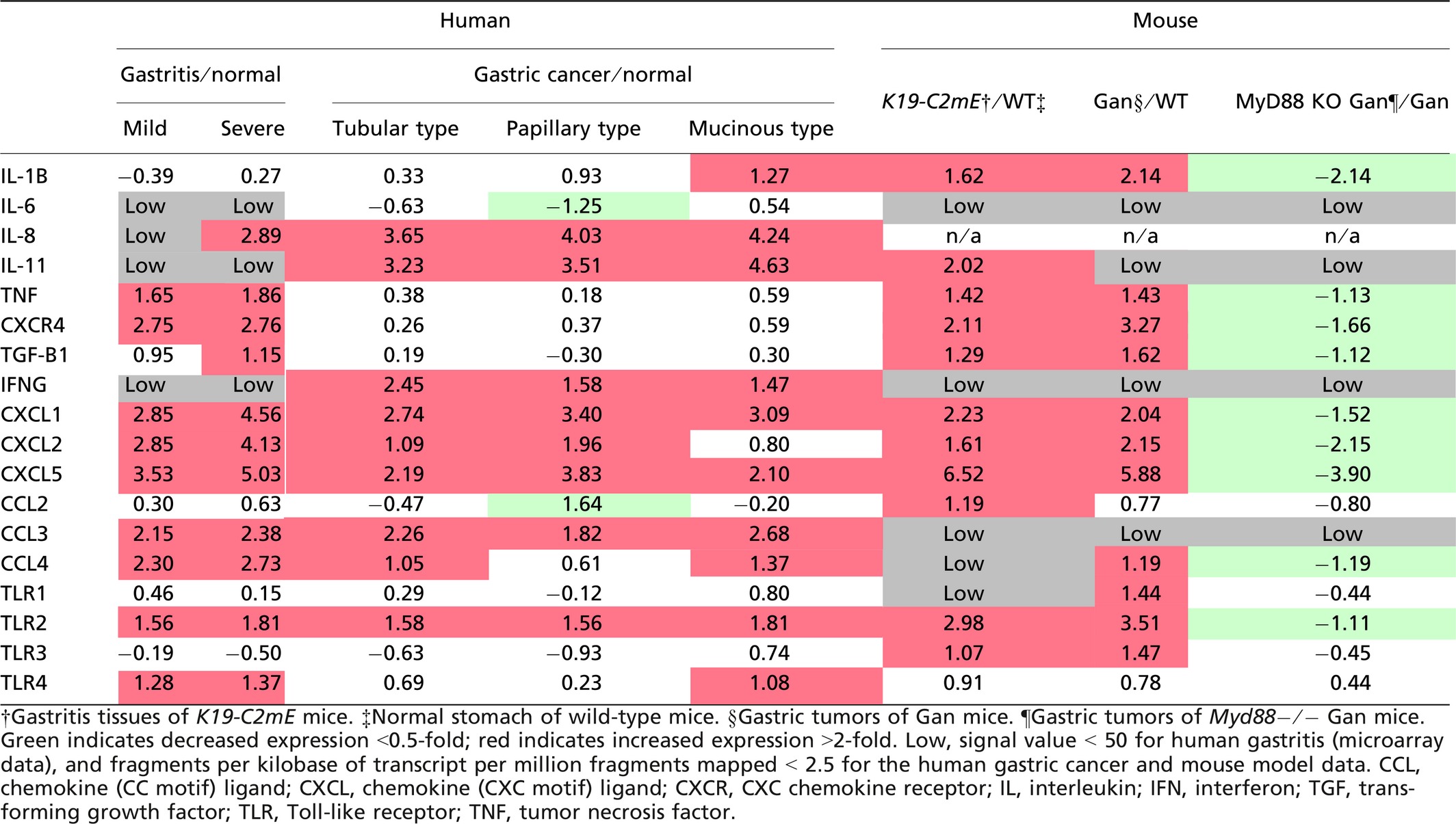
Interleukin-11 signaling by the gp130 receptor promotes gastric tumorigenesis through Stat3 activation.23, 52 Moreover, PGE2 induces expression of CXCL1 and CXCL2, which further induces colitis-associated colon cancer through recruitment of CXC chemokine receptor 2-expressing MDSCs.23 A similar mechanism was shown for CXCL5, which recruits TGF-β-expressing MDSCs to tumor tissues, resulting in malignant progression by immune suppression and the M2-type host reaction.53 Accordingly, both “infection-associated” and “tumor-elicited” inflammation may promote gastric tumorigenesis through induction of tumor-promoting cytokines, and the COX-2/PGE2 pathway is involved in this induction process.
Toll-like receptor signaling in generation of inflammatory microenvironment
Inflammation is triggered when innate immune cells detect infection or tissue injury through TLRs.54 As described above, TLR/MyD88 signaling plays a role in the generation of the tumor inflammatory microenvironment.14 Moreover, disruption of the MyD88 gene (Myd88) in mouse models suppressed sporadic intestinal tumors,55, 56 colitis-associated colon cancer,57, 58 gastric cancer,59 liver cancer,60 skin tumors, and sarcomas.61 Notably, disruption of Myd88 in ApcMin mice suppressed expression of COX-2 and other cytokines.55 These results suggest that TLR/MyD88 signaling promotes tumorigenesis through induction of the COX-2/PGE2 pathway, which is required for generation of the inflammatory microenvironment (Fig. 3).
Importantly, however, we recently found that disruption of Myd88 in the BMDCs of Gan mice significantly suppressed gastritis and gastric tumors,16 and that cytokine expression levels were dramatically decreased, even though the COX-2/PGE2 pathway was stably activated (Table 1). Accordingly, it is possible that the cooperation of the COX-2/PGE2 and TLR/MyD88 pathways is required for generation of the inflammatory microenvironment (Fig. 3).
In intestinal tumor tissues, epithelial barrier function is impaired, and thus microbial products can stimulate the TLRs of myeloid-derived cells to express IL-23, which further activates Th17 response.62 Consistently, expression of IL-23 and IL-17 is upregulated in Gan mouse tumors in a MyD88-dependent manner.16 It has been shown that IL-17 expression is required for inflammatory response and intestinal polyposis in ApcMin mice.63 Such a mechanism may activate the TLRs of BMDCs in gastric cancer tissues (Fig. 3).
“Cell-intrinsic” role of TLR/MyD88 signaling in tumorigenesis
It has been shown that TLR2 and TLR4 signals through MyD88 are important for intestinal mucosal homeostasis and regeneration.64 Moreover, NF-κB signaling in epithelial cells promotes inflammation-associated intestinal tumorigenesis.8 These results suggest a “cell-intrinsic” role of TLR/MyD88 signaling through NF-κB activation in epithelial homeostasis and tumorigenesis.
Importantly, TLR2/MyD88 signaling in intestinal epithelial cells plays a critical role in expansion of the stem cell pool in damaged mucosa and is required for tumor growth in mouse models.65 Interestingly, TLR2 is the most upregulated TLR in gastric tumors in both human and mouse models (Table 1),66 and its expression is predominantly detected in tumor epithelial cells.16 Notably, disruption of the TLR2 gene (Tlr2) in a gp130F/F gastric tumor mouse model in which the hyperactivation of Stat3 causes gastric tumorigenesis,67 results in significant suppression of tumor formation without inhibition of inflammation.66 This suggests that TLR2/MyD88 signaling in tumor cells does not contribute to the inflammatory microenvironment but that it is important for tumorigenesis through the maintenance of stemness of tumor cells by a cell-intrinsic mechanism (Fig. 3). Expression of TLR2 has consistently been reported to be associated with maintenance of cancer stem cells in mammary and ovarian cancer.68, 69
Expression of TLR2 is also induced in gastritis of both humans and mouse models, and its expression in Gan mouse tumors was significantly decreased by disruption of Myd88.16 Moreover, it has been shown that Stat3 regulates TLR2 expression in gastric cancer cells.66 It is therefore conceivable that TLR2 expression is induced in tumor cells by IL-11 signaling through gp130/Stat3 pathway, which is activated in the inflammatory microenvironment of gastric tumors.
How does TLR2/MyD88 regulate stemness in tumor cells? It has been reported that activation of NF-κB together with Wnt signaling promotes acquisition of stem cell properties, inducing dedifferentiation of intestinal epithelia.70 Accordingly, it is possible that activation of TLR2/MyD88 signaling through NF-κB, together with Wnt signaling, increases self-renewal activity of gastric tumor cells (Fig. 3). Activation of the NOX1/ROS pathway by TNF-α and the cytokine network may also contribute to the cell-intrinsic role of TLR2/MyD88 signaling in regulation of stemness as described above.
The ligands that are responsible for activation of TLR signaling in BMDCs and tumor epithelial cells is a matter of interest. In gastric cancer, H. pylori is an important ligand to activate TLRs, but other tumor cell-derived factors, such as versican or high mobility group box 1 are other possible candidates for TLR activation as damage-associated molecular patterns;71, 72 however, this remains to be investigated.
Conclusions
It has been established that the COX-2/PGE2 pathway plays a tumor-promoting role through generation of the inflammatory microenvironment. Maintenance of stemness is one of the important COX-2/PGE2-dependent tumor promotion mechanisms. It has also been shown that TLR/MyD88 signaling is important for tumorigenesis through activation of innate immunity, which also induces generation of the inflammatory microenvironment. As discussed in this review, the interplay of these two distinct pathways is required for generation of the inflammatory microenvironment, which has a critical role in tumor promotion. Moreover, it has been shown that the cell-intrinsic role of epithelial TLR2/MyD88 signaling is important for tumor cell stemness and gastric tumorigenesis, and such innate immune responses in tumor cells are activated by the inflammatory microenvironment (Fig. 3). Accordingly, targeting the COX-2/PGE2 pathway together with TLR/MyD88 signaling may suppress the inflammatory microenvironment as well as the stemness of gastric tumor cells and could be an effective preventive and therapeutic strategy against gastric cancer.
Acknowledgments
We thank Dr. Yoichi Yamada for discussions about bioinformatics, and Ms. Manami Watanabe and Ms. Ayako Tsuda for their excellent technical assistance in Gan mouse studies.
Disclosure Statement
The authors have no conflicts of interest.
Abbreviations
-
- BMDC
-
- bone marrow-derived cell
-
- CCL
-
- chemokine (CC motif) ligand
-
- COX
-
- cyclooxygenase
-
- CXCL
-
- chemokine (CXC motif) ligand
-
- IL
-
- interleukin
-
- MDSC
-
- myeloid-derived suppressor cell
-
- NOX1
-
- NADPH oxidase 1
-
- NF-κB
-
- nuclear factor-κB
-
- PG
-
- prostaglandin
-
- PGE2
-
- prostaglandin E2
-
- ROS
-
- reactive oxygen species
-
- Stat
-
- signal transducer and activator of transcription
-
- TCGA
-
- The Cancer Genome Atlas
-
- TNF-α
-
- tumor necrosis factor-α
-
- TLR
-
- Toll-like receptor




