Downregulation of WDR20 due to loss of 14q is involved in the malignant transformation of clear cell renal cell carcinoma
Funding Information:
This work was supported in part by Grants in-Aid for Scientific Research (to M. M.;15K08405) from the Japanese Ministry of Education, Culture, Sports Science and Technology.
Abstract
Previously, we reported that genomic loss of 14q occurs more frequently in high-grade than in low-grade clear cell renal cell carcinomas (ccRCCs), and has a significant impact on the levels of expression of genes located in this region, suggesting that such genes may be involved in the malignant transformation of ccRCCs. Here, we found that six of the genes located in the minimal common region of 14q loss were significantly downregulated in high-grade ccRCCs due to copy number loss. Using a dataset from The Cancer Genome Atlas Research Network, we found that downregulation of one of these six genes, WDR20, was significantly associated with poorer outcome in patients with ccRCC, suggesting that WDR20 downregulation may be involved in the malignant transformation of ccRCCs. In functional assays, exogenous WDR20 significantly inhibited the growth of RCC cell lines and induced apoptosis. Interestingly, the phosphorylation levels of ERK and protein kinase B/AKT, which reportedly contribute to the malignant phenotype of RCC cells, were clearly reduced by exogenous expression of WDR20. Thus, our data suggest that downregulation of WDR20 due to 14q loss may be involved in the malignant transformation of ccRCCs, in part through activation of the ERK and protein kinase B/AKT pathways.
Abbreviations
-
- AKT
-
- protein kinase B
-
- ccRCC
-
- clear cell renal cell carcinoma
-
- mTOR
-
- mammalian target of rapamycin
-
- PHLPP1
-
- PH domain and leucine-rich repeat protein phosphatase 1
-
- PI3K
-
- phosphatidylinositol 3-kinase
-
- RCC
-
- renal cell carcinoma
-
- TCGA
-
- The Cancer Genome Atlas
-
- UAF1
-
- ubiquitin-specific peptidase 1-associated factor 1
-
- USP
-
- ubiquitin-specific peptidase
Renal cell carcinoma (RCC) accounts for approximately 3% of all adult malignancies and 90–95% of all kidney neoplasms.1 Clear cell RCC (ccRCC) is the most common histological subtype, accounting for approximately 70–80% of RCC cases.1, 2 Among the various clinicopathological features of ccRCC, nuclear grading, also known as the Fuhrman grading,3 is the most useful prognostic parameter. Patients with nuclear grades 3 and 4 (high-grade) ccRCC have been reported to show a poorer survival rate than those with nuclear grades 1 and 2 (low-grade) ccRCC.3
Previously, to reveal the alterations associated with the malignant transformation of ccRCC, we analyzed the genomic alterations in 26 cases of ccRCC (11 low-grade and 15 high-grade). We found that 14q loss occurred more frequently in high-grade than in low-grade ccRCCs, and that many of the genes located on 14q were downregulated due to copy number loss.4 Recently, using a relatively large cohort (246 cases), Klatte et al.5 also showed that 14q loss was associated with a higher TNM stage, higher nuclear grade, larger tumor size, and poorer prognosis. It has also been reported that 14q loss is associated with poorer survival,5-8 a higher Fuhrman grading5, 6 and distant metastasis.5 These data from our group and others5-8 suggest that 14q loss is involved in the malignant transformation of ccRCC through downregulation of tumor suppressor genes located in this region. However, the mechanism by which 14q loss affects the malignant transformation of ccRCC remains largely unknown.
In the present study, to identify the gene(s) responsible for 14q loss from among those located in the minimal common region involved, 14q32.31-33, determined in our previous study,4 we compared the expression of these genes between low- and high-grade ccRCCs and analyzed the effect of downregulation of the candidate genes on prognosis by using the ccRCC dataset from The Cancer Genome Atlas (TCGA). Furthermore, we examined the possible tumor-suppressive function of WDR20, whose expression was downregulated in high-grade ccRCC due to copy number loss at14q and associated with poorer prognosis.
Materials and Methods
Details of array data analysis, vector construction, establishment of stable cell lines, removal of integrated cDNA, isolation of cells by cell sorting, RNA extraction, quantitative RT-PCR, invasion assay, gene expression microarray analysis, treatment of cells with 5-aza-2′-deoxycytidine and trichostatin A, and migration assay are provided in Document S1.
Patients
Primary tumors of RCC were surgically resected at Oita University Hospital and Oita Red Cross Hospital (Oita, Japan) and diagnosed histopathologically as described previously4, 9 (Table S1). Use of the tissue samples for all experiments was approved by the Oita University Ethics Committee (approval no. P-05-05).
Dataset analysis
Datasets pertaining to mRNA expression, DNA copy number, and clinical information for 499 ccRCC patients from the TCGA Research Network (http://cancergenome.nih.gov/) were analyzed using cBioPortal for Cancer Genomics (http://www.cbioportal.org).10, 11 The patients were considered to have high and low expression of each candidate gene based on Z scores of >0 and <−2, respectively. Also, the genomic copy number status of each candidate gene was determined using Affymetrix (Santa Clara, CA, USA) SNP6 array-based GISTIC analysis, in which values of −2, −1, 0, 1, and 2 represent putative homozygous deletion, hemizygous deletion, no change, gain, and high-level amplification, respectively.
Cell lines
The RCC cell lines 786-O and 769-P were purchased from ATCC (Manassas, VA, USA) and maintained in accordance with the supplier's instructions.
Western blot analysis
Western blotting was carried out as described previously with slight modifications.12 The primary antibodies were: anti-FLAG M2 (Sigma-Aldrich, St. Louis, MO, USA), anti-p-AKT S473 D9E, anti-pan AKT, anti-p-ERK, anti-total ERK (Cell Signaling Technology, Danvers, MA, USA), anti-WDR20 (Bethyl, Montgomery, TX, USA), and anti-GAPDH (Sigma-Aldrich). Detection was carried out with ECL Prime Western Blotting Detection Reagents (Amersham Biosciences, Piscataway, NJ, USA) in accordance with the manufacturer's instructions. GAPDH was used as an internal control.
Proliferation assay
We cultured 1 × 103 cells of each cell line in 96-well plates for 0, 48, 72, and 96 h, and measured the viabilities of the cells using the CellTiter96 aqueous one solution cell proliferation assay (Promega, Madison, WI, USA) in accordance with the manufacturer's instructions.
Colony formation assay
For stably transfected cell lines, 100 cells were cultured in 10-cm dishes for 14 days. For transiently transfected cells, 1 × 105 cells were cultured in a 10-cm dish and selected with G418 (1.2 mg/mL) for 14 days. The resulting colonies were fixed with 10% buffered formalin, stained with 0.05% toluidine blue solution (pH 7.0) (Wako, Osaka, Japan) and quantified.
Apoptosis assay
We evaluated the induction of apoptosis by measuring cytoplasmic oligonucleosomal fragments and caspase 3/caspase 7 activities. Cytoplasmic oligonucleosomal fragments of the cells cultured for 72 h in a 96-well plate were measured with a Cell Death Detection ELISAPLUS kit (Roche Applied Science, Mannheim, Germany) in accordance with the manufacturer's instructions. Activities of caspase 3 and 7 in the cells cultured for 96 h in a 96-well plate were determined using a caspase-Glo 3/7 assay kit (Promega) in accordance with the manufacturer's instructions.
Statistical analysis
Differences in the expression levels of the candidate genes between low- and high-grade ccRCCs were determined by the Wilcoxon test and Tukey–Kramer test. Associations between the expression levels of candidate genes and patient survival were expressed by Kaplan–Meier curves and analyzed by log–rank test in cBioPortal. Differences in quantitative RT-PCR, proliferation, colony formation, invasion, migration, and apoptosis assays were analyzed by two-sided Student's t-test. Values are expressed as mean ± SEM. Differences at P values of <0.05 were considered to be statistically significant.
Results
Genomic loss of 14q is associated with downregulation of WDR20, BAG5, ZFYVE21, ZNF839, and C14orf79 in high-grade ccRCCs
To identify genes located in the minimal common region of 14q loss and downregulated due to copy number loss, we compared the gene expression profiles of ccRCCs with and without 14q loss. Among the genes located on 14q32.31-33, six showed an expression level of <0.75 in cases with 14q loss relative to those without 14q loss (Table S2). In addition, we found that five of these six genes were significantly downregulated in high-grade relative to low-grade ccRCCs while the remaining gene showed no difference, suggesting that downregulation of these five genes, WDR20, BAG5, ZFYVE21, ZNF839, and C14orf79, may be related to malignant transformation of ccRCC (Table S2, Figs 1, S1).
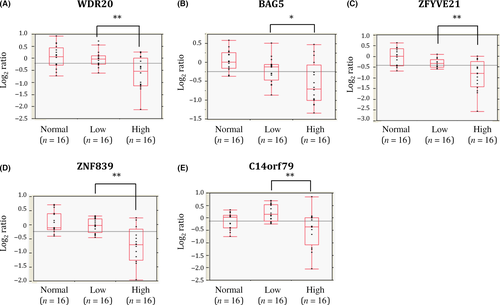
Downregulation of WDR20 due to copy number loss is related to poorer outcome of patients with ccRCCs
Next, we analyzed the associations between downregulation of each of the above five genes and patient survival using a TCGA dataset.13 Although downregulation of four of the five genes had no correlation with prognosis, a lower level of WDR20 expression was significantly associated with a poorer outcome (Figs 2A,S2). As shown in Figure 2(B), we also found that patients with WDR20 gene copy number loss showed a poorer outcome than those without such loss. Furthermore, the copy number of WDR20 was strongly correlated with the level of its mRNA (Fig. 2C). These results suggest that downregulation of WDR20 due to genomic copy number loss is associated with poorer survival of patients with ccRCCs. The expression level and copy number of WDR20 were also significantly associated with T stage, lymph node metastasis, and distant metastasis in the TCGA dataset (Table S3). A significant association between WDR20 expression and T stage was also observed in our dataset (Fig. S3). Additionally, our dataset revealed a tendency for an association between WDR20 expression and vascular involvement, although this did not reach statistical significance (P = 0.0975, Fig. S3).
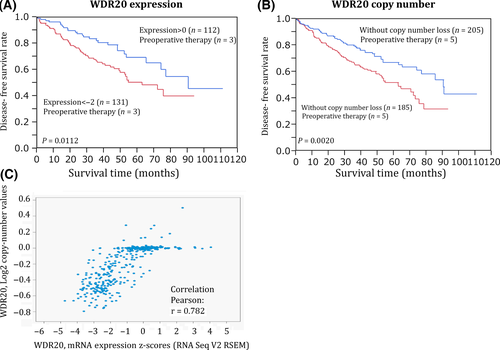
Although almost all ccRCCs with 14q loss showed single-copy genomic loss of WDR20 as judged by array CGH analysis,4 mutational inactivation of the remaining WDR20 allele appeared unlikely, as somatic mutation of WDR20 was not detected at all in the TCGA dataset. However, treatment with a combination of the DNA demethylating agent 5-aza-dC and the histone deacetylase inhibitor trichostatin A (TSA) resulted in slight but statistically significant upregulation of WDR20 (Fig. S4), suggesting that epigenetic modifications may be partly involved in silencing of WDR20 on the remaining allele in ccRCC with 14q loss (Fig. S4).
Exogenous expression of WDR20 inhibits cell proliferation and induces apoptosis in RCC cells
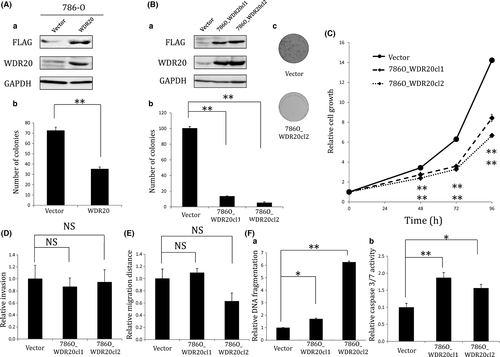
To determine whether expression of WDR20 regulates the proliferation of RCC cells, we transfected 786-O cells, which show hemizygous loss and downregulation of WDR2014(Fig. S5), with a plasmid encoding FLAG-tagged WDR20 cDNA and carried out colony formation assays. Overexpression of WDR20 in the transfected cells was confirmed by Western blotting (Fig. 3Aa). As shown in Figure 3(Ab), exogenous WDR20 significantly inhibited the colony-forming ability of 786-O. Next, we established two 786-O cell lines, 786O_WDR20 cl1 and 786O_WDR20 cl2, stably expressing WDR20 (Figs 3Ba,S6), and carried out colony formation assays. Both cell lines formed fewer colonies and showed a slower growth rate than control cells (Figs 3Bb,c,C). The growth-suppressive function of WDR20 was confirmed by using another RCC cell line, 769-P, showing hemizygous loss and downregulation of WDR2014(Fig. S5). We established two cell lines, 769P_WDR20 cl1 and 769P_ WDR20 cl2, stably expressing WDR20 in 769-P. Despite only a slight increase in the level of WDR20 mRNA in the established cells (Fig. S7A), their growth was suppressed, as assessed by MTS assay (Fig. S7B). We noticed that significant inhibition of cell viability by WDR20 took longer to appear in 769-P than in 786-O cells. This may have been due to the difference in the growth speed and level of ectopically expressed WDR20 between the two cell lines. We also investigated the effect of WDR20 expression on RCC cell invasiveness, motility, and apoptosis. Overexpression of WDR20 had little effect on the invasiveness and motility of 786-O cells (Fig. 3D,E), but significantly induced apoptosis as assessed by detection of cytoplasmic oligonucleosomal fragments or activated caspase 3/7 (Fig. 3F). Taken together, our results suggest that expression of WDR20 inhibits the growth of RCC cells partly through induction of apoptosis.
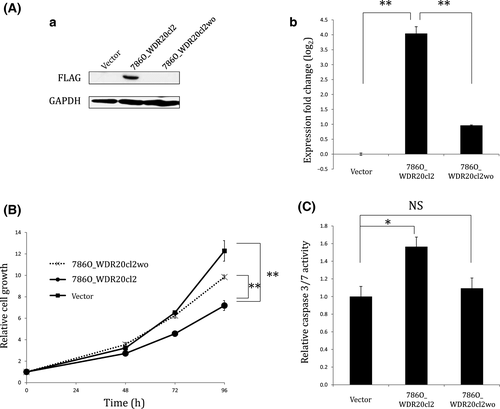
To confirm the growth-suppressive and apoptosis-inducing effect of WDR20, we removed cDNA of WDR20 from its stable transfectant, 786O_WDR20 cl2, using transposase, and established a subclone, 786O_WDR20 cl2wo, in which expression of WDR20 was substantially decreased at both the protein and mRNA levels (Fig. 4A). As shown in Figure 4(B,C), we found that the growth rate inhibition and apoptosis induction in 786O_WDR20 cl2 were reduced to almost the same levels as those in the parental 786-O cells by removal of WDR20 cDNA, confirming that the growth inhibition and apoptosis induction in 786O_ WDR20 cl2 were caused by the ectopically expressed WDR20.
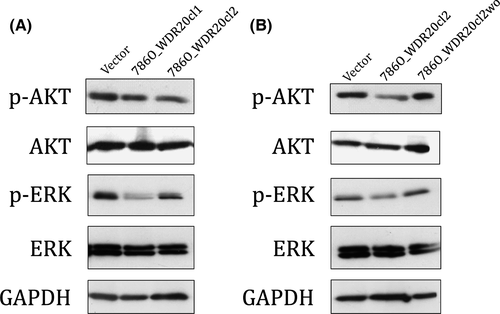
Expression of WDR20 suppresses phosphorylation levels of AKT and ERK
To determine the mechanism by which WDR20 inhibits cell growth and induces apoptosis, we analyzed the effect of WDR20 overexpression on the activities of the RAF/MEK/ERK and PI3K/AKT/MTOR pathways using Western blotting. As shown in Figure 5, exogenous WDR20 suppressed the phosphorylation of both ERK and AKT, whereas removal of WDR20 cDNA reduced this suppression to the same level as that in the parental cells, suggesting that the level of WDR20 expression is associated with activation of both ERK and AKT. We also found that several of the genes related to the RAF/MEK/ERK and/or PI3K/AKT/MTOR pathways were differentially expressed by the ectopic expression of WDR20 (Table S4). Therefore, we concluded that growth inhibition and apoptosis induction by WDR20 are due, at least partly, to suppression of the ERK and AKT pathways.
Discussion
To identify genes whose downregulation affects the malignant characteristics of ccRCC, we focused on the minimal common region of 14q loss, which we had defined previously as 14q32.31-33.4 Among the genes located in the minimal common region, WDR20, BAG5, ZFYVE21, ZNF839, and C14orf79 were significantly downregulated in high-grade ccRCC due to copy number loss. Although upregulation of BAG5 and ZFYVE21 is reportedly associated with apoptosis resistance in prostate cancer15 and metastasis of colorectal carcinoma,16 respectively, it has not yet been determined whether downregulation of any of these genes is related to tumor malignancy. In the present study, we showed for the first time that a lower level of WDR20 expression was significantly associated with poorer outcome in patients with ccRCC. Although we cannot exclude the possibility that the other five genes also have tumor-suppressive roles in ccRCC, our data suggest that downregulation of WDR20 due to 14q loss contributes to the malignant transformation of ccRCC.
In this study, we found that overexpression of WDR20 induced growth suppression and apoptosis in ccRCC cells. WDR20 is a member of the WD (tryptophan-aspartate) repeat family of proteins that have four to 16 WD domains, each of which possesses a conserved core sequence comprising approximately 40 amino acids flanked by a GH (glycine–histidine) dipeptide at the N-terminus and a WD dipeptide at the C-terminus.17 It has been reported that WDR20, which has five WD domains, is a component of the deubiquitinase complex and stimulates the activities of two deubiquitinating enzymes, ubiquitin-specific peptidase (USP)12 USP46, cooperatively with USP1-associated factor 1 (UAF1).18 Although there are no reports about the tumor-suppressive role of WDR20, the involvement of USP12 and USP46 in tumorigenesis has been reported previously. USP12 is known to deubiquitinate histones H2B and H2A and induce apoptosis when overexpressed in prostate cancer cells.19 USP12 has also been reported to inhibit the growth of prostate cancer cells by deubiquitinating PHLPP1 that dephosphorylates and inactivates AKT.20, 21 In addition, in colon cancer, it has been reported that USP46 plays a role in inhibiting the activation of AKT through deubiquitination of PHLPP1 in coordination with WDR20 and USP1-associated factor 1.22 Therefore, together with these previous reports, our data suggest that WDR20 might exert its tumor-suppressive function through regulation of deubiquitinating enzymes, such as USP12 and USP46. Further studies are required to clarify the mechanism whereby WDR20 induces growth suppression and apoptosis in ccRCC cells.
In the present study, we found that overexpression of WDR20 suppressed the phosphorylation of AKT and ERK. Aberrant activation of the PI3K/AKT/MTOR and RAF/MEK/ERK pathways is frequently observed in various types of cancer, including ccRCC, and enhances the proliferation or survival of cancer cells.13, 23, 24 In ccRCC, enhanced activation of the PI3K/AKT/MTOR pathway is frequently observed, and this is correlated with local invasion, regional lymph node involvement, microscopic vascular invasion, distant metastasis, and poor prognosis.25, 26 At the same time, it has been reported that a high level of phospho-ERK is frequently observed in ccRCC and correlated with poor prognosis, tumor size, invasiveness, and clinical stage.23, 27 Interestingly, molecular targeted agents that block aberrant activation of the PI3K/AKT/MTOR and/or RAF/MEK/ERK pathways, such as MTOR and multiple receptor tyrosine kinase inhibitors, are currently used for first-line treatment of ccRCC, suggesting that activation of these pathways is the driver of ccRCC.28-30 Thus, our data suggest that WDR20 suppresses the growth of ccRCC cells, at least partly, through inhibition of these driver pathways. Further studies to clarify the mechanism whereby WDR20 regulates the phosphorylation of AKT and ERK might provide a much better understanding of feasible therapeutic strategies for ccRCC.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (to M.M., no. 15K084051) from the Japanese Ministry of Education, Culture, Sports Science and Technology. We thank Noriko Hamamatsu, Saiko Kato, and Mami Kimoto for their excellent technical assistance.
Disclosure Statement
The authors have no conflict of interest.




