Sensitive cytometry based system for enumeration, capture and analysis of gene mutations of circulating tumor cells
Funding Information:
This work was supported in part by Advanced Research and Development Project on Diagnosis and Treatment for Early Stage of Cancer, Development of Automatic Testing System for Genetic Diagnosis using Peripheral Blood from New Energy and Industrial Technology Development Organization, and by a Grant-in-Aid for the Third-Term Comprehensive 10-Year Strategy for Cancer Control of Japan including Awardee of Research Resident Fellowship from the Foundation for Promotion of Cancer Research (Japan).
Abstract
Methods for the enumeration and molecular characterization of circulating tumor cells (CTC) have been actively investigated. However, such methods are still technically challenging. We have developed a novel epithelial cell adhesion molecule independent CTC enumeration system integrated with a sorting system using a microfluidics chip. We compared the number of CTC detected using our system with those detected using the CellSearch system in 46 patients with various cancers. We also evaluated epidermal growth factor receptor (EGFR) and PIK3CA mutations of captured CTC in a study of 4 lung cancer and 4 breast cancer patients. The percentage of samples with detected CTC was significantly higher with our system (65.2%) than with CellSearch (28.3%). The number of detected CTC per patient using our system was statistically higher than that using CellSearch (median 5, 0; P = 0.000172, Wilcoxon test). In the mutation analysis study, the number of detected CTC per patient was low (median for lung, 4.5; median for breast, 5.5); however, it was easy to detect EGFR and PIK3CA mutations in the CTC of 2 lung and 1 breast cancer patient, respectively, using a commercially available kit. Our system is more sensitive than CellSearch in CTC enumeration of various cancers and is also capable of detecting EGFR and PIK3CA mutations in the CTC of lung and breast cancer patients, respectively.
Circulating tumor cells (CTC) originate from a primary solid tumor and are found transiting the circulatory system.1 CTC are typically defined as nucleated cells lacking CD45 expression and expressing epithelial marker proteins, such as epithelial cell adhesion molecules (EpCAM) or cytokeratins. Enumeration and characterization of CTC are, as a “liquid biopsy,” expected to provide useful clinical information on prognosis, cancer staging, drug choice and therapeutic efficacy. Indeed, accumulating evidence suggests that CTC isolation from a blood sample may allow reliable early detection and molecular characterization of cancer at diagnosis, and may provide a minimally invasive method to guide and monitor the results of cancer therapy.2, 3 The US Food and Drug Administration approved EpCAM-dependent CTC enumeration system (CellSearch [Veridex, Raritan, NJ, USA]) has prognostic value for patients with various cancers.4-7 However, there remain issues of sensitivity and difficulties in molecular analysis of CTC.
We have developed a novel CTC enumeration flow-cytometry-based system, FISHMAN-R, and a CTC capture system, On-chip Sort (On-Chip Biotechnologies, Tokyo, Japan), which are platforms independent of EpCAM expression. On-chip Sort is a cell sorter that is integrated with FISHMAN-R. We recently reported that FISHMAN-R is highly efficient in the detection of spiked rare cells in peripheral blood8 and that On-chip Sort is capable of capturing spiked rare cells efficiently from peripheral blood in a condition that is satisfactory for mutation analysis.9
Based on the above data, for the present study, we conducted prospective clinical studies regarding the detection of CTC in patients with various cancers. In these studies, we compared our system using FISHMAN-R with the CellSearch system for the enumeration of CTC. We also investigated the suitability of On-chip Sort CTC capture for the analysis of EGFR mutations of advanced lung cancer patients and of PIK3CA mutations of advanced breast cancer patients in captured CTC using commercially available mutation detection kits, which have been approved in the USA, Japan and China, and are also in compliance with the European Directive 98/79/EC.
Material and Methods
Buffer, reagents and antibodies
BD Pharm Lyse lysing solution and Lyse/Fix Buffer were purchased from BD Biosciences (San Jose, CA, USA). The FcR blocking reagent and CD45 Microbeads (Dynabeads, DYNAL) were purchased from Miltenyi Biotec (Bergisch-Gladbach, Germany) and Invitrogen (Carlsbad, CA, USA), respectively. The FITC-conjugated anti-cytokeratin (CK) mAb CK3-6H5, phycoerythrin (PE)-conjugated anti-CD326 (EpCAM) mAb 9C4, and Alexa Fluor 700-conjugated anti-CD45 mAb F10-89-4 were purchased from Miltenyi Biotec, BioLegend (San Diego, CA, USA) and AbD Serotec (Oxford, UK), respectively.
Cell culture
Non-small cell lung cancer cell lines H1650, H1975, PC-9 and PC-14, and breast cancer cell line BT20 were used. PC-9 and PC-14 were kindly provided by Professor Hayata of Tokyo Medical College (Tokyo, Japan). H1650, H1975 and BT20 were obtained from the American Type Culture Collection (Manassas, VA, USA). The cells were cultured in RPMI-1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 10% FBS (GIBCO, Life Technologies, Grand Island, NY, USA), penicillin, streptomycin and amphotericin B (10 000 U/mL, 10 mg/mL and 25 μg/mL, respectively [Sigma-Aldrich]) under a humidified atmosphere containing 5% CO2 at 37°C.
Circulating tumor cell enumeration and capture instrumentation
The novel multicolor flow-cytometry-based CTC detection and capturing system of this article utilized FISHMAN-R (On-Chip Biotechnologies) and On-Chip Sort (On-Chip Biotechnologies), which is a cell-sorter integrated with FISHMAN-R. These devices use a disposable microfluidics chip and include a collection reservoir within which the sorted cells are stored (Fig. 1a–c). This design enables reduction of sample loss to the absolute minimum; specifically, the dead volume of analysis using this chip is <0.01 μL. This system therefore provides suitable conditions for the enumeration of rare cells such as CTC from patients' blood. The details of the FISHMAN-R and On-Chip Sort were described previously.8-10 Data analysis was performed using FlowJo software v7.6.5 (Tree Star, Ashland, OR, USA). The principle of sorting is described on the manufacturer's website (http://www.on-chip.co.jp/en/index.html).
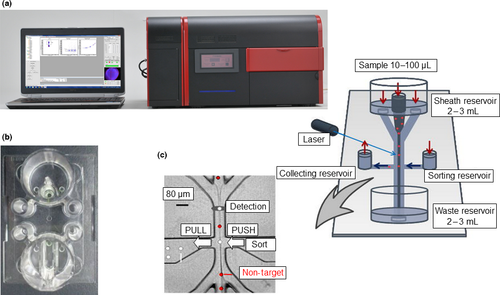
Circulating tumor cell enrichment, immunofluorescent staining process and enumeration
Circulating tumor cell enrichment from 4 mL of peripheral blood includes hemolysis and elimination of leukocytes by using CD45 antibody-conjugated microbeads. After the enrichment process, the CTC-enriched samples were subsequently fixed and stained using anti-CK-FITC, anti-EpCAM-PE and anti-CD45-Alexa Fluor 700 antibodies. Then, the samples were applied to FISHMAN-R or On-chip Sort. A flowchart of this process including the CTC enrichment percentage is shown in Figure 2.
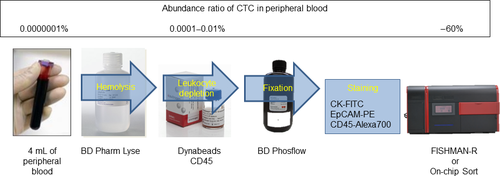
Our system defined CTC as CK+ and/or EpCAM+ and CD45− cells, with a diameter comparable to cell size. The parameter forward scatter/side scatter threshold was preset to allow detection of particles greater than approximately 6–8 μm in size so as to eliminate particles other than CTC, such as unbound microbeads and cell fragments.
Circulating tumor cells sorting and gene analysis of captured cells
Circulating tumor cell sorting was performed using On-chip Sort. DNA was isolated from sorted CTC by using the QIAamp DNA Micro Kit (QIAGEN, Hilden, Germany). Isolated DNA was analyzed using the Therascreen EGFR RGQ PCR kit and the PI3K Mutation Test kit, which are based on ARMS and Scorpions PCR technologies (QIAGEN). The Therascreen EGFR RGQ PCR kit amplifies and analyzes mutations in the EGFR gene including 19 deletions in exon 19 and amino acid changes of T790M in exon 20 or L858R in exon 21. The PI3K Mutation Test Kit amplifies and analyzes the PIK3CA gene, including mutations in exons 9 and 20, which lead to E542K, E545K or H1047R amino acid changes.
Patient measurements and blood sample collection in the circulating tumor cell enumeration study
We analyzed 46 patients with various cancers and 5 healthy donors. To determine the threshold level of our method, we also analyzed 13 healthy donor samples. Healthy donors were defined as persons who had never been diagnosed with cancer before study enrollment. These healthy donor samples were collected and analyzed in three independent institutions. All patients and healthy donors in the present studies below provided informed consent and their participation in the studies was approved by the institutional review committee of the National Cancer Center. This work was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Ten milliliters of peripheral blood was collected in a CellSave tube for CTC enumeration by CellSearch. A total of 5 mL of blood was collected in a standard 5-mL blood collection tube containing EDTA, and 4 mL of this blood was used for CTC enumeration by FISHMAN-R. The CellSave tube was delivered to an independent medical laboratory (GeneticLab, Sapporo, Japan), where CTC were enumerated using a CellSearch CTC Kit. To confirm the superiority of FISHMAN-R over CellSearch in detecting EpCAM-negative cells, 18 cells of the EpCAM-negative lung cancer cell line PC-14 were spiked into the blood of a healthy volunteer and the sample was analyzed in the same manner. In this study, all samples were analyzed by investigators who were blinded to the sample origin.
Method validation of circulating tumor cell sorting and mutation analysis in spiked blood samples
We previously reported that spiked-in rare tumor cells in blood can be sorted using On-chip Sort independent of EpCAM expression.9 To confirm the efficiency of On-chip Sort in capturing rare cells and to estimate the cell number threshold for mutation detection, we performed spike-in experiments using lung and breast cancer cell lines before the clinical trial.
Various numbers of PC-9 cells and BT-20 cells were picked up using a micropipette under an inverted microscope and were then added to a blood sample (4 mL). After these spiked samples were processed using our CTC enrichment method, the spiked cells were captured using On-chip Sort. The number of captured cells was visually counted under a fluorescence microscope, and we then calculated the sorting efficiency. To estimate the cell number threshold for detection of EGFR deletion mutations or the PIK3CA variant H1047R mutation in sorted cells, various numbers of PC-9 cells, H1650 cells and BT20 cells were spiked into 4 mL of blood, and the spiked cells were captured using On-chip Sort. Mutations in the captured cells were then analyzed using the gene analysis method described in the subsection above titled “Circulating tumor cells sorting and gene analysis of captured cells.”
Patient measurements and blood sample collection in the circulating tumor cells sorting and mutation analysis study
We analyzed 4 lung cancer and 4 breast cancer patients. The EGFR mutation status had been confirmed in surgical or biopsied specimens of the lung cancer patients before enrolment in this study. Breast cancer patients were enrolled without testing PIK3CA mutation status. Peripheral blood (4 mL) for this study was drawn from the study participants. CTC sorting using On-chip Sort and gene mutation analyses of captured cells were performed as described in the subsection above titled “Circulating tumor cells sorting and gene analysis of captured cells.”
Statistical analysis
EZR (Saitama Medical Center, Jichi Medical University), which is a graphical user interface for R (The R Foundation for Statistical Computing, version 2.13.0), was used for statistical analyses.11
Results
Circulating tumor cell enumeration study
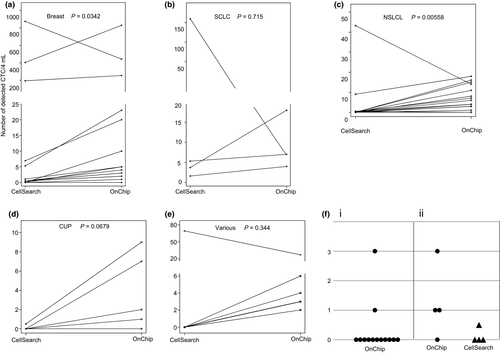
We compared the sensitivity of our system with that of CellSearch for the detection of CTC in the blood of patients with various types of cancers. Eighteen patients with non-small cell lung cancer, 4 with small cell lung cancer, 13 with breast cancer, 5 with cancer of unknown primary, 3 with cervical cancer, 1 with peritoneum cancer, 1 with endometrial cancer and 1 with head and neck cancer were enrolled. Patient clinical data and the number of CTC detected in each patient examined using FISHMAN-R and CellSearch are shown in Table 1. The result of 13 healthy donor samples determined the threshold level of our system as 3 CTC/4 mL (Fig. 3f[i]). The number of CTC detected per patient using FISHMAN-R ranged from 0 to 829 (median, 5)/4 mL, and the CTC number of 65.2% (30/46) of the samples was above the threshold level. In contrast, using CellSearch, the CTC number of only 28.3% (13/46) of the samples was greater than the threshold level determined in a previous study (≥2/7.5 mL).1 When the blood sample of a healthy donor that was spiked with 18 EpCAM-negative PC-14 cells was analyzed, while FISHMAN-R detected 10 of the spiked cells, CellSearch did not even detect a single spiked cell. In all of the other blood samples from the healthy donors, the number of CTC detected by FISHMAN-R and CellSearch was not greater than the threshold of each system (Fig. 3f[ii]). The results showed that our system was significantly more sensitive than that of the CellSearch system for the detection of CTC in breast cancer and non-small cell lung cancer patients (P = 0.0342 and 0.00558, Wilcoxon test; Fig. 3a,c). Although not reaching a level of statistical significance, the CTC detection sensitivity in the other cancer types, including small cell lung cancer and cancer with unknown primary, showed a trend toward being greater than that of the CellSearch system (Fig. 3b,d,e).
| Pt | Primary | Histology | Stage | Age/Sex | Metastatic site | FISHMAN-R/4 mL | CellSearch/4 mL | CellSearch/7.5 mL |
|---|---|---|---|---|---|---|---|---|
| 1 | Lung | SCLC | IIIB | 79/M | ― | 7 | 159.5 | 299 |
| 2 | Lung | SCLC | IIIB | 59/F | ― | 18 | 3.7 | 7 |
| 3 | Lung | SCLC | IV | 61/M | Liver, bone | 4 | 1.6 | 3 |
| 4 | Lung | SCLC | IV | 66/M | Liver | 7 | 5.3 | 10 |
| 5 | Lung | NSCLC (Sq) | IIIB | 66/M | ― | 0 | 0 | 0 |
| 6 | Lung | NSCLC (Sq) | IV | 59/F | Retroperitoneum | 0 | 0.5 | 1 |
| 7 | Lung | NSCLC (Sq) | IV | 74/M | Liver, bone | 8 | 0 | 0 |
| 8 | Lung | NSCLC (Sq) | IV | 59/M | Lung, LN | 18 | 9.1 | 17 |
| 9 | Lung | NSCLC (Ad) | IIIA | 66/M | ― | 1 | 0 | 0 |
| 10 | Lung | NSCLC (Ad) | IIIB | 63/F | ― | 6 | 0 | 0 |
| 11 | Lung | NSCLC (Ad) | IV | 61/F | Lung | 3 | 0 | 0 |
| 12 | Lung | NSCLC (Ad) | IV | 54/M | Brain, Liver, kidney, bone, lung, pleura | 4 | 0 | 0 |
| 13 | Lung | NSCLC (Ad) | IV | 54/M | Brain, adrenal, LN (abdominal) | 4 | 0.5 | 1 |
| 14 | Lung | NSCLC (Ad) | IV | 61/F | Lung, liver | 4 | 0 | 0 |
| 15 | Lung | NSCLC (Ad) | IV | 68/F | Lung | 7 | 0.5 | 1 |
| 16 | Lung | NSCLC (Ad) | IV | 50/F | LN | 8 | 0 | 0 |
| 17 | Lung | NSCLC (Ad) | IV | 51/M | Pleura | 11 | 0 | 0 |
| 18 | Lung | NSCLC (Ad) | IV | 73/F | Bone | 14 | 43.2 | 81 |
| 19 | Lung | NSCLC (Ad) | IV | 61/F | Brain | 15 | 0 | 0 |
| 20 | Lung | NSCLC (Ad) | IV | 75/M | Lung | 16 | 0 | 0 |
| 21 | Lung | NSCLC (other) | IV | 63/M | ― | 3 | 0 | 0 |
| 22 | Lung | NSCLC (LCNEC) | IV | 71/M | LN (neck) | 3 | 0 | 0 |
| 23 | Breast | IDC | IIIc | 72/F | ― | 5 | 0 | 0 |
| 24 | Breast | ILC | IV | 53/F | LN, liver, bone, stomach | 0 | 0 | 0 |
| 25 | Breast | IDC | IV | 45/F | Lung, liver, bone | 1 | 0 | 0 |
| 26 | Breast | Ad | IV | 74/F | Bone | 2 | 0.5 | 1 |
| 27 | Breast | IDC | IV | 58/F | LN, lung, bone | 3 | 0.5 | 1 |
| 28 | Breast | IDC | IV | 58/F | LN, lung, liver, bone, skin | 4 | 0.5 | 1 |
| 29 | Breast | IDC | IV | 42/F | Lung, bone, pleura | 5 | 1.1 | 2 |
| 30 | Breast | IDC | IV | 71/F | Bone, pleura | 10 | 0 | 0 |
| 31 | Breast | IDC | IV | 56/F | Liver, bone, stomach | 20 | 6.9 | 13 |
| 32 | Breast | IDC | IV | 35/F | LN, lung, liver, bone | 23 | 23 | 23 |
| 33 | Breast | IDC | IV | 45/F | LN, bone | 255 | 193.6 | 363 |
| 34 | Breast | IDC | IV | 60/F | Lung, liver, bone, brain | 443 | 874.7 | 1640 |
| 35 | Breast | IDC | IV | 70/F | Bone, pleura | 829 | 402.7 | 755 |
| 36 | Cervix | Sq | IV | 54/F | Bone | 3 | 3 | 0 |
| 37 | Cervix | Sq | IV | 60/F | LN | 4 | 0 | 0 |
| 38 | Cervix | Sq | IV | 47/F | LN, lung | 6 | 0 | 0 |
| 39 | Peritoneum | Ad | IIIc | 72/F | Peritoneum | 3 | 0 | 0 |
| 40 | Endometrium | Ad | IV | 66/F | Peritoneum | 2 | 0 | 0 |
| 41 | CUP | Mesothelioma | IV | 61/F | Lung, peritoneum, pleura, subcutaneous | 0 | 0 | 0 |
| 42 | CUP | Ad | IV | 46/M | LN | 1 | 0 | 0 |
| 43 | CUP | Ad | IV | 43/M | LN, brain | 2 | 0 | 0 |
| 44 | CUP | Ad | IV | 68/M | LN, lung | 7 | 0 | 0 |
| 45 | CUP | Ad | IV | 66/M | LN, lung, brain, adrenal, thyroid | 9 | 0.5 | 1 |
| 46 | Head and Neck | SCC | IV | 61/M | LN, liver, bone, bone marrow | 24 | 71.5 | 134 |
| Healthy donor | 1 | 0 | 0 | |||||
| Healthy donor | 1 | 0 | 0 | |||||
| Healthy donor | 0 | 0 | 0 | |||||
| Healthy donor | 3 | 0.5 | 1 | |||||
| Healthy donor | 18 cells of PC-14 were spiked in blind | 10 | 0 | 0 |
- Ad, adenocarcinoma; CUP, cancer of unknown primary; F, female; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; LCNEC, large cell neuroendocrine carcinoma; LN, lymph node; M, male; NSCLC, non-small cell lung cancer; Pt, patient number; SCLC, small cell lung cancer; Sq, squamous cell carcinoma; TKI, tyrosine kinase inhibitor; -, no metastases.
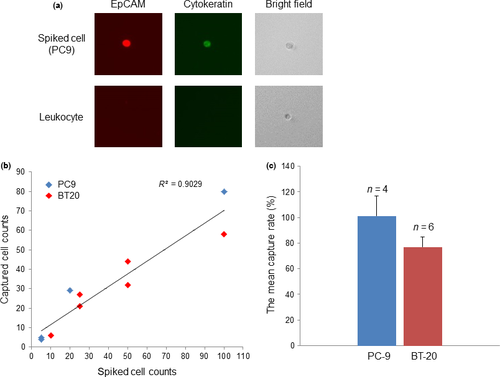
Sorting efficiency and mutation analysis of sorted cells in spiked blood samples
PC-9 cells (5, 20 or 100 cells) and BT-20 cells (10, 25, 50 or 100 cells) were spiked into 4 mL of peripheral blood, and the spiked cells were then recovered from the blood using On-chip Sort. Recovered cells were visually distinguished from leukocytes (Fig. 4a) and were then counted under a fluorescent microscope. Regression analysis of the number of captured cells versus the number of spiked cells produced a correlation coefficient (R2) of 0.9029 (Fig. 4b). The mean capture rates were 101.35 ± 15.3% for PC-9 and 77.0 ± 8.1% for BT-20 (Fig. 4c). To estimate the minimal number of CTC that are required for detection of mutations using our method, we performed further spike-in experiments. PC-9 cells (5, 10 or 100 cells) and H1650 cells (5, 10 or 30 cells), which have the EGFR exon 19 deletion mutation, and BT20 cells (10, 20 or 50 cells) that express the PIK3CA variant H1047R mutation were spiked into 4 mL of peripheral blood. Following processing of each sample in a manner similar to that described for CTC enrichment (Fig. 2), the spiked cells were sorted from the peripheral blood using On-chip Sort, which successfully sorted spiked cells from all samples. EGFR deletion mutation was detected in samples spiked with 5, 10 or 100 PC-9 cells and 5, 10 or 30 H1650 cells using the method described in the Materials and Methods. The PIK3CA variant H1047R mutation was detected in samples spiked with 20 or 50 BT20 cells. The same results were obtained twice in independent experiments.
Circulating tumor cell sorting and mutation analysis study
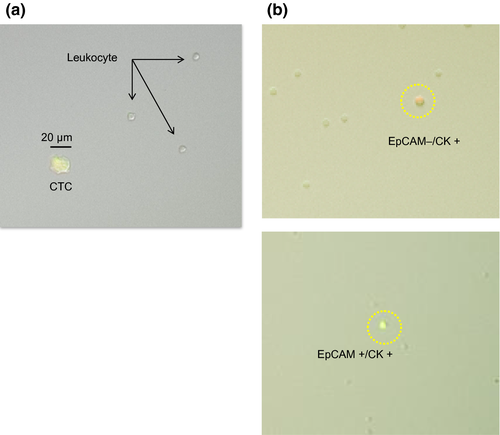
The patient clinical data and the number of CTC detected in each patient in the CTC mutation analysis study are shown in Table 2. A typical isolated CTC with a few leukocytes is shown in Figure 5a. In some cases, EpCAM-negative/CK-positive CTC could be detected (Fig. 5b). EGFR deletion mutation was detected in 2 of 4 lung cancer patients and the PIK3CA variant H1047R mutation was detected in 1 of 4 breast cancer patients using PCR of the captured CTC (Table 2). Archival primary tumor tissue of the patient in whom the PIK3CA mutation was detected in captured CTC was analyzed for PIK3CA mutation; however, the same mutation was not detected.
| Primary | Histology | Stage | Age/Sex | Metastatic site | Mutation Status | EGFR TKI | Detected CTC /4 mL | Mutation detected in captured CTC |
|---|---|---|---|---|---|---|---|---|
| Lung | NSCLC (Ad) | IV | 74/F | ― | Exon 19 deletion | Untreated | 3 | None |
| Lung | NSCLC (Ad) | IV | 45/F | LN, liver, bone, stomach | Exon 19 deletion | Treated | 2 | None |
| Lung | NSCLC (Ad) | IV | 75/F | Lung, liver, bone | Exon 19 deletion | Treated | 20 | Exon 19 deletion |
| Lung | NSCLC (Ad) | IV | 76/F | Bone | Exon 19 deletion | Treated | 6 | Exon 19 deletion |
| Breast | IDC | IV | 64/F | LN, lung, bone | Unknown | ― | 4 | None |
| Breast | IDC | IV | 77/F | LN, lung, liver, bone, skin | Unknown | — | 7 | None |
| Breast | IDC | IV | 75/F | Lung, bone, pleura | Unknown | — | 3 | None |
| Breast | IDC | IV | 71/F | Bone, pleura | Unknown | — | 11 | PIK3CA (H1047R) in exon 20 |
- Ad, adenocarcinoma; F, female; IDC, invasive ductal carcinoma; LN, lymph node; NSCLC, non-small cell lung cancer.
Discussion
We developed a novel multicolor flow cytometry-based CTC detection system, “FISHMAN-R,” and a CTC capture system, “On-chip Sort.” The detection rate of CTC in patients with various cancers using FISHMAN-R was 65.2% (n = 46), in contrast with 28.3% using CellSearch. In almost all of the samples, our system detected more CTC than CellSearch. However, there were some cases in which CellSearch detected many more CTC than our system. In those cases (Nos. 1, 33, 34, 35), the size of most of the CTC detected using CellSearch was 4–6 um. Our system failed to enumerate these CTC because, based on previous studies, we assumed that the size of a CTC is larger than 6 μm.12-14 Therefore, in the present study, the lower limit of CTC detection size was set by reference to these previous data. Thus, the lower CTC counts obtained using our system compared with CellSearch possibly result from the difference in the size threshold. Other current CTC detection methodologies have reported that the size of CTC is larger than that detected using CellSearch in the present study.15, 16 Optimization of the size threshold in our system in order to reduce CTC counting loss is a problem that remains to be solved.
Several studies have shown that the presence of EpCAM on tumor cells varies with tumor type.17 It has also been suggested that the low prevalence of CTC detected in patients with advanced non-small cell lung cancer using the CellSearch system may be due to the loss of EpCAM expression.18 Furthermore, a previous study suggested that EpCAM expression may limit CTC detection efficiency between technologies, especially in patients with metastatic lung cancer.19 Indeed, using our system we found that EpCAM-negative/CK-positive CTC, which could not be detected by CellSearch, can be detected in some cases, including in non-small cell lung cancer. Regarding the CTC detection rate in non-small cell lung cancer patients, our system showed a significantly higher rate than that of CellSearch (66.7 vs 11.1%). Such a discrepancy in the detection rate between our system and that of CellSearch indicates that our CTC detection system is superior to that of CellSearch due to its EpCAM independence. It has previously been reported that EpCAM-negative/CK-positive cells are often observed in disseminated tumor cells in bone marrow after chemotherapy.20 In addition, it has been reported that there is a type of CTC that co-expresses CK and mesenchymal components, the so-called EMT state, during the development of cancer.21 From the perspective of our results, cytokeratin (including epithelial marker-based detection) independent of EpCAM expression has the potential to detect more EMT cells or chemo-resistant cells than EpCAM-dependent detection. Additional staining of mesenchymal components in those cells may reveal the nature of those cells.
In the CTC sorting and mutation analysis study of lung and breast cancer patients, CTC were captured by On-chip Sort in 7 of 8 cases and it was possible to detect EGFR and PIK3CA mutations in the captured CTC. It is now clear that EGFR mutations have significant value for tailoring individualized EGFR tyrosine kinase inhibitor therapy in lung cancer.22 Moreover, the clinical importance of PIK3CA mutations is growing, with many phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR inhibitors currently being tested in preclinical and clinical settings.23-25 Therefore, easy analysis of such gene mutations of therapeutic targets is urgently needed. However, the availability of tumor specimens for testing of mutation status in the clinical setting is often limited. Moreover, there are currently considerable problems with invasive methods for obtaining tumor specimens and technical issues regarding tumor biopsy. A substitute approach for obtaining tumor specimens is the analysis of CTC, which enables tumor specimens to be easily obtained. CTC analysis can also be easily repeated, and might allow real-time monitoring of drug-sensitive and resistant mutations in individual patients.26 Because it remains unclear whether the CTC that are detected in blood are representative of all cancer cells in the body, further research will be required to determine the significance of the mutations detected in CTC. In the very limited number of patients that were available to us, the results of mutation analysis of the CTC indicated that concordance between CTC and primary tissue in EGFR and PIK3CA mutation status was quite low. There has been a report of discrepancies in the EGFR mutation detection rate between primary tissue and CTC obtained by CellSearch when ultra-deep sequencing was used in mutational analysis.27 Similarly, discordance between primary tumor tissue and CTC in PIK3CA status has been reported28 The low concordance rate between CTC and primary tissue in mutation detection that we obtained may be due to the low number of CTC, considering the results of our preclinical experiments; however, our result could also reflect CTC heterogeneity in mutations, as previously reported.29
In 17 samples from healthy donors, our system detected 3 CTC in 2 samples and 1 CTC in 3 samples. There is still room for improvement in the specificity of CTC detection in our system. More samples of healthy donors should be evaluated to define a reliable threshold.
In summary, the results of clinical feasibility studies showed that our system is more sensitive than that of CellSearch for CTC enumeration in various cancers. In addition, our system is useful for the detection of EGFR and PIK3CA mutations in the captured CTC of lung and breast cancer patients, respectively, using commercially available kits. Our CTC capture and analysis approach may be used as a noninvasive repeatable biopsy method. It is desirable to further evaluate this system in the future using more clinical samples.
Disclosure Statement
Yuu Fujimura is a salaried employee of On-Chip Biotechnologies. The other authors have no conflict of interest to declare.




