Cancer immunotherapy using novel tumor-associated antigenic peptides identified by genome-wide cDNA microarray analyses
Funding Information:
Japan Society for Promotion of Science; Ministry of Education, Culture, Sports, Science and Technology, Japan; OncoTherapy Science, Inc.
Abstract
Recent genome-wide cDNA microarray analysis of gene expression profiles in comprehensive tumor types coupled with isolation of cancer tissues by laser-microbeam microdissection have revealed ideal tumor-associated antigens (TAAs) that are frequently overexpressed in various cancers including head and neck squamous cell cancer (HNSCC) and lung cancer, but not in most normal tissues except for testis, placenta, and fetal organs. Preclinical studies using HLA-transgenic mice and human T cells in vitro showed that TAA-derived CTL-epitope short peptides (SPs) are highly immunogenic and induce HLA-A2 or -A24-restricted CTLs. Based on the accumulated evidence, we carried out a phase II clinical trial of the TAA-SP vaccine in advanced 37 HNSCC patients. This study showed a significant induction of TAA-specific CTLs in the majority of patients without serious adverse effects. Importantly, clinical responses including a complete response were observed in this study. Another phase II clinical trial of therapeutic TAA-SP vaccine, designed to evaluate the ability of prevention of recurrence, is ongoing in HNSCC patients who have received curative operations. Further studies in human preclinical studies and in vivo studies using HLA class I transgenic mice showed TAA-derived long peptides (TAA-LPs) have the capacity to induce not only promiscuous HLA class II-restricted CD4+ T helper type 1 cells but also tumor-specific CTLs through a cross-presentation mechanism. Moreover, we observed an augmentation of TAA-LP-specific T helper type 1 cell responses and tumor antigen-spreading in HNSCC patients vaccinated with TAA-SPs. This accumulated evidence suggests that therapeutic TAA-SPs and LPs vaccines may provide a promising cancer immunotherapy.
Immunotherapy has become an epoch-making and attractive therapeutic modality for cancer, which offers potentially targeted therapy with fewer adverse-effects compared with conventional therapy.1 One type of immunotherapy is a checkpoint blockade therapy2 using humanized mAbs specific to CTL antigen 4 (CTLA-4),3, 4 programmed cell death -1 (PD-1),5 or its ligand PD-L1.6 This therapy induced remarkable and durable clinical responses in patients with melanoma, lung, renal, and bladder cancers, even though the patients were at advanced stages with systemic metastasis. CTLA-4, PD-1, and PD-L1 are involved in immune suppression of tumor-reactive T cells, and blockades of these molecules by antibody enhance mainly T-cell-mediated immune response to tumor. These observations give us an important message that tumor-bearing hosts can mount an endogenous antitumor immune response to eliminate cancer cells. Therefore, a combination of active immunization with tumor-associate antigens (TAAs) in various forms and immune checkpoint blockades is one promising cancer immunotherapy to be challenged.
Rosenberg et al.7 reported that the clinical effect of vaccination of TAA-derived CTL-epitope peptides (short peptides; SPs) alone was limited in a very small fraction of advanced melanoma patients. However, we attempted to overcome various problems and discovered novel and ideal TAAs. Recently, we obtained a promising clinical result from a phase II clinical trial of a novel TAA-SP vaccine in advanced head and neck squamous cell cancer (HNSCC) patients. In this clinical study, a mixture of multiple CTL-epitope SPs derived from novel TAAs were used as reported by Walter et al.8 and we showed that the SP vaccine had clinical effects in advanced cancer patients. Further investigation of therapeutic SP vaccine for cancer immunotherapy is ongoing in clinical trials to treat several cancer types. Here we introduce recent developments of new TAA–peptide-based cancer immunotherapy and provide an overview of current challenges and future directions of this immunotherapy.
Characteristics of an Ideal TAA Useful for Cancer Immunotherapy
The following three characteristics are required for an ideal TAA to elicit effective and safe T-cell-mediated antitumor immunity in cancer patients.
- Cancer-specific expression of TAA. An ideal TAA must be frequently overexpressed in cancer tissues, but must not be expressed in normal tissues except for fetal organs and testis. Cancer–testis antigen (CTA) and oncofetal antigen (OFA) are ideal TAAs because immunization of cancer patients with these TAAs is expected not to induce serious autoimmune diseases, but to induce effective tumor immunity.
- Oncogenic characteristics of TAA. It is well known that tumors escape from host antitumor immunity by various mechanisms such as a loss of HLA class I molecule or TAA expression. In terms of loss of TAA expression, TAA involved in oncogenesis is considered to be barely lost in the process of tumor progression. Thus, oncogenic TAA is an ideal TAA.
- Immunogenicity of TAA. This third crucial characteristic demands that TAA must have the capacity to induce an immune response in cancer patients. These immune responses can be evaluated by detection of TAA-specific immunoglobulin or TAA-specific T-cell responses such as γ-interferon (IFN-γ), tumor necrosis factor-α (TNF-α), and interleukin-2 (IL-2) secretion by T cells.
Genome-wide cDNA Microarray Analysis as a Powerful Tool to Identify Ideal TAAs
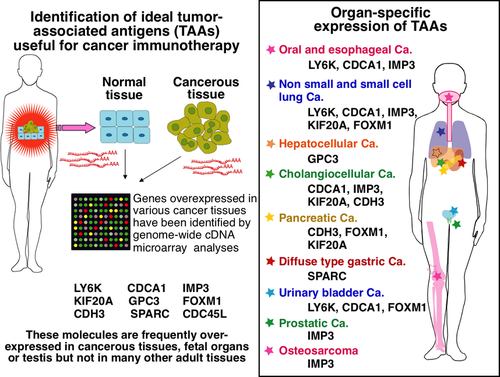
Many methods have been developed to identify TAAs. Among them, a genome-wide cDNA microarray analysis is one of the powerful and promising methods. This technology provides us with comprehensive information of gene expression profiles in both normal9 and cancer tissues.10-14 The data allowed us to identify ideal TAAs such as CTAs and OFAs as targets of cancer immunotherapy. Y. Nakamura and his colleagues have found various TAAs that have ideal characteristics as targets for cancer immune therapy, such as cancer-specific expression and oncogenic features, using genome-wide cDNA microarray analysis coupled with isolation of cancer cells by laser-microbeam microdissection. Those TAAs have been shown to be frequently overexpressed in various cancer tissues including hepatocellular,10 pancreatic,11 lung,12, 13 esophageal,13, 14 urinary bladder cancers, HNSCC, and several other cancers, and have characteristics of CTA or OFA, as shown in Figure 1.
Our strategy to identify TAA-derived and Japanese common HLA-A2 (A*02:01) or HLA-A24 (A*24:02)-restricted CTL epitopes is as follows (shown in Fig. 2). First, we used several well-established computer algorithms that predict SPs consisting of 9 or 10 amino acids possibly bound by HLA-A2 or A24 molecules. We then synthesized the predicted SPs and tested their immunogenicity using HLA-A2 or A24-transgenic mice, which are gene-manipulated mice to express HLA-A2 or A24 transgenes but not express mouse endogenous MHC class I genes.15, 16 Subsequently, human PBMCs isolated from healthy donors and cancer patients were used to check immunogenicity. We stimulated human CD8+ T cells with the SPs selected by in vivo screening study using HLA transgenic mice to identify immunogenic SPs that can activate HLA-A2 or A24-restricted human CTLs. This strategy allowed us to identify many novel TAA-derived immunogenic CTL-epitope SPs in various cancer types including oral, esophageal,17-20 lung,18-22 gastric,23 hepatocellular,24 and pancreatic25-27 cancers. In some newly identified SPs, the antitumor activities of SP-specific CTLs were also observed in humanized mouse in vivo studies. These studies used an immunocompromised mouse that was inoculated with human cancer cells expressing TAAs and either HLA-A2 or A24. Then TAA-SP-specific human CTLs generated in vitro from purified CD8+ T cells by stimulation with cognate peptides were intravenously injected.17, 21, 23-25
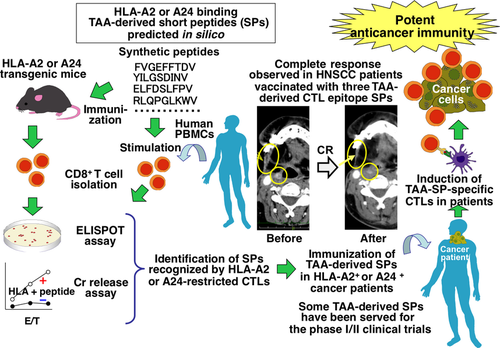
Phase I/II Clinical Trials of CTL-epitope SP-based Cancer Immunotherapy
Kono et al.28, 29 and Iwahashi et al.30 have conducted phase I and phase II clinical studies of TAA-SP-vaccine immunotherapy for advanced esophageal cancer. These studies showed induction of TAA-specific CTL responses and significant correlation between antigen-specific CTL induction and improved progression-free survival. Suzuki et al.31 showed that a mixture of therapeutic peptide-vaccine derived from novel CTAs and vascular endothelial growth factor receptor 1 and 2 was safe and induced peptide-specific CTL responses in advanced/recurrent non-small-cell lung cancer patients. In this phase I study, 47% of patients exhibited stable disease. The median overall survival (OS) time and 2-year survival rates were 398 days and 32.8%, respectively.
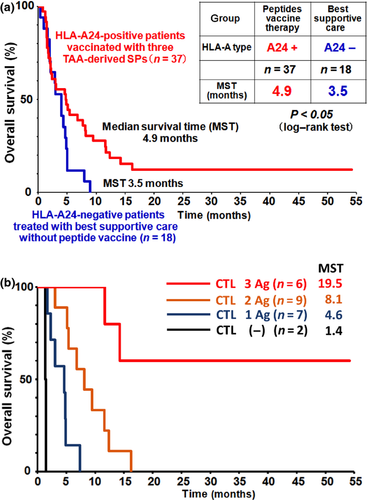
Recently, Yoshitake et al.32, 33 reported that a mixture of therapeutic peptide-vaccines derived from three different CTAs, namely lymphocyte antigen 6 complex, locus K (LY6K), cell division cycle associated 1 (CDCA1), and insulin-like growth factor-II mRNA binding protein 3 (IMP-3), induced TAA-specific CTLs and antitumor activity in advanced HNSCC patients. In this trial, a mixture of three SPs was given subcutaneously and weekly with incomplete Freund's adjuvant to a total of 37 patients with advanced HNSCC. CTA-specific CTL responses in PBMCs were also assessed using Enzyme-Linked ImmunoSpot assay and HLA-A24-SP multimer assay. This cancer immunotherapy study was well tolerated in patients with advanced HNSCC and no serious adverse effects were observed. Importantly, the OS of the HLA-A24-positive vaccinated patients (n = 37) was significantly longer than that of the HLA-A24-negative patients (n = 18) who received the best supportive care (Fig. 3a). The median survival time was 4.9 months in vaccinated patients, whereas it was 3.5 months in non-vaccinated patients (P < 0.05). In addition, a noteworthy result was observed in this clinical trial; an advanced HNSCC patient showed a complete response. In the HLA-A24-positive vaccinated patients, the peptide-specific CTL responses were observed for LY6K-SP in 85.7% of patients, for CDCA1-SP in 64.3% of patients, and for IMP-3-SP in 42.9% of patients. Interestingly, there is a positive correlation between better clinical responses and the number of CTA-SPs to which the patients exhibited CTL responses (Fig. 3b). Namely, vaccinated patients who had CTL responses to all three CTA-SPs showed significantly improved clinical outcomes compared to patients who had CTL responses to two CTA-SPs or only one SP. These results suggest that the CTA-specific CTL response induced by therapeutic SP vaccine may be a predictive marker of HNSCC patients treated with TAA-SP vaccine immunotherapy.
Important Role of CD4+ T Helper Type 1 Cells in Induction of Effective Tumor Immunity
It has been reported that vaccination of CTL-epitope SPs alone is not enough to induce effective antitumor immunity. All nucleated cells express MHC class I molecules, therefore the SPs composed of 9 or 10 amino acids can be presented to naive CD8+ T cells by any nucleated cells, which are non-professional antigen-presenting cells, thus do not express co-stimulatory molecules such as CD80/86. T cell receptor signaling without co-stimulation leads to anergy of CD8+ T cells.34, 35 Other studies in mice have shown that immunization of TAA-SPs with incomplete Freund's adjuvant induced retention of SP-specific CTLs at the sites of immunization and CTLs became dysfunctional and underwent antigen-driven apoptosis, thus tumor-reactive CTLs did not migrate into tumor sites.36 Synthetic long peptides (LPs) encompassing CTL-epitopes can overcome such weaknesses of SP-based immunotherapy, at least in part. Dendritic cells (DCs), as potent professional antigen-presenting cells, can cross-present SPs embedded in LPs, and have the capability to activate SP-specific naive CD8+ T cells. In addition, LPs have the potential to bind to HLA class II molecules and activate the LP-specific T helper (Th) cells. Therefore, LP-based immunotherapy has a potential to generate both tumor-specific CTLs and Th cells, which may provide long-lasting antitumor effects.
T helper cells have been shown to play a crucial role in antitumor immune responses.37-39 These cells are essential for priming of tumor-specific naive CD8+ T cells to induce the proliferation and differentiation of tumor-specific CTLs,40 the generation and maintenance of long-lasting memory CTL responses, and for entry of CTLs into the tumor microenvironment.41 Among various subsets of Th cells, Th1 cells play a crucial role in elimination of tumors. IFN-γ produced by Th1 cells has been shown to have anti-angiogenic effects in tumor microenvironments. Tumor-reactive Th1 cells also induce activation of DCs, natural killer cells, and type 1 macrophages to combat against tumor cells. In cancer patients, it has been shown that Th1 cell responses to TAAs were associated with better prognosis.42-44
Dendritic cells have a unique capacity to process proteins and LPs, which are engulfed in endosomes, into SPs. Then DCs present SPs in the context of HLA class I molecules through the cross-presentation pathway to activate TAA-SP-specific CTLs. Thus, it is reasonable to discover single-chain LPs harboring both multiple Th1 cell-epitopes and CTL-epitopes SPs to induce strong antitumor immunity by activating both TAA-specific Th1 cells and CTLs. Therefore, TAA-derived LPs may be a promising candidate for cancer immunotherapy.
Identification of TAA-derived LPs Activating both TAAs-specific Th1 cells and CTLs
To identify TAA-derived LPs (TAA-LPs), which induce TAA-specific Th1 cells and CTLs, we combined in silico data obtained from a recently developed computer algorithm,45 which predicts peptide sequences bound to HLA class II molecules,46 and TAA-derived CTL-epitopes SPs (Fig. 4). First, we focused on three novel CTAs, LY6K,47 CDCA1,48 and Kinesin family member 20A (KIF20A).49 These TAA-SPs have been identified as highly immunogenic in preclinical studies and in phase I/II clinical trials. The peptide-vaccine immunotherapies of advanced HNSCC and pancreatic cancer with TAA-SPs (LY6K, CDCA1, IMP3, and KIF20A-derived CTL-epitope SPs) have shown that vaccination with TAA-SPs induced the appearance of TAA-SPs-specific and HLA-A24-restricted CTLs in patients' peripheral blood. These studies also demonstrated the monitoring of TAA-specific CTLs by tetramer assay and IFN-γ Enzyme-Linked ImmunoSpot assay was feasible in peripheral blood. As shown in Table 1, we identified highly immunogenic TAA-derived 20–26-mer LPs that can activate Th1 cells, which are restricted by various HLA class II molecules frequently observed in the Japanese population.47-49 These TAA-LPs are possibly applicable to many Japanese patients to induce Th cells.
| TAA-derived Th cell epitope LPs | Amino acids numbers | HLA class II alleles encoding for restriction molecules of Th cells | Cytokines produced | Presence of Th cells in patients vaccinated with SPs | Cross-presentation to CTLs | Restriction HLA-class I of CTLs specific to SPs included in LPs |
|---|---|---|---|---|---|---|
|
LY6K (119–142) LP |
24 |
DRB1*08:03/15:02, DPB1*05:01 |
Th1-type | + ~ ++ | NT | NT |
|
LY6K (172–191) LP |
20 |
DRB1*15:02, DQ (unknown) |
Th1-type | ++ | Yes | A24 |
|
CDCA1 (55–78) LP |
24 |
DRB1*04:05/15:02, DPB1*02:01 |
Th1-type | +++ | Yes | A2/A24 |
|
CDCA1 (39–64) LP |
26 | DRB1*09:01/15:02 | IFN-γ only tested | +++ | Yes | A24 |
|
KIF20A (60–84) LP |
25 |
DRB1*15:02, DPB1*02:01 |
Th1-type | + | Yes | A24 |
|
KIF20A (809–833) LP |
25 |
DRB1*15:02, DRB4*01:03 (DR53) |
Th1-type | + | Yes | A2 |
- We identified highly immunogenic TAA-derived 20–26-mer LPs that can activate Th1 cells restricted by HLA-class II molecules often found in Japanese populations in healthy donors and cancer patients.47-49 Natural processing of TAA-LPs that can activate Th1 cells generated by stimulation with synthetic LPs, and cross-presentation to CTLs of short peptides (SPs) derived from LPs by dendritic cells were also proved in many LPs. Head and neck squamous cell carcinoma patients showed increased immune responses of TAA-LP-specific Th cells after vaccination with TAA-SPs. NT, not tested.
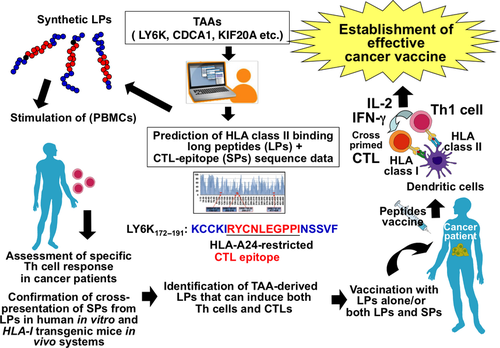
These TAA-specific bulk Th cell lines and clones generated by stimulation with TAA-LPs produced large amounts of Th1 cytokines including IL-2, IFN-γ, and TNF-α and little Th2 cytokines such as IL-4, IL-5, and IL-13 in recognition of the cognate LPs presented by HLA class II molecules, suggesting the TAA-LPs can induce Th1 cells and may generate Th1-poralized immunity in cancer patients. In addition, the LP-specific and promiscuous HLA class II-restricted Th1 cells responded to DCs loaded with recombinant human TAA proteins, indicating that LPs encompass multiple Th1-epitopes naturally processed by DCs. Dendritic cells may engulf TAAs, which are released from dead cancer cells in the tumor microenvironment, present Th1-epitopes carrying similar sequences embedded in TAA-LPs in the context of HLA class II molecules, and activate tumor-specific Th1 cells in cancer patients. Our recent studies also showed that TAA-LP-induced Th1 cells express the cytotoxicity marker CD107a after stimulation with cognate LPs, suggesting that TAA-LP-induced Th1 cells have the potential to kill cancer cells that express HLA class II and TAAs.
Importantly, we showed that cross-presentation of LPs efficiently primes TAA-SP-specific CTLs in vitro in a human T-cell culture system and in vivo in HLA-A2 or A24 transgenic mice. The TAA-LP-pulsed human DCs induced efficient expansion of TAA-specific human CTLs in vitro. However, the IFN-γ production of antigen-specific CTLs were completely abrogated by fixation of DCs with paraformaldehyde, suggesting that DCs engulf and process TAA-LPs into SPs to cross-present CTL-epitopes embedded in the LPs to antigen-specific CTLs. We also found that vaccination with CDCA1-derived LPs more efficiently induced CDCA1-specific CTLs, which recognize a CTL-epitope embedded in LP, compared with SP (CTL-epitope) vaccination in HLA-A24 transgenic mice, suggesting that CDCA1-LP vaccination may be superior to SP vaccination in vivo. In this study, the synergistic effect of TAA-LPs on induction of TAA-SP-specific CTLs was investigated. In a human in vitro culture system, TAA-specific CTLs were generated efficiently from CD8+ T cells in the presence of TAA-SP + TAA-LP + LP-specific Th cells compared with CD8+ T cells cultured with TAA-SP alone, indicating that simultaneously activated Th1 cells augment generation of tumor-specific CTLs from naive CD8+ T cells.47-49
The frequency of TAA-LP-specific Th1 cells before and after SP vaccine immunotherapy has been studied in patients with HNSCC, who were enrolled in a phase II trial of immunotherapy using three CTA-derived CTL-epitope SP vaccines. The LP-specific Th1 cell responses were clearly observed in HNSCC patients before and after SP vaccination.47-49 Cancer patients mounted more TAA-LP-specific Th1 cell responses compared with healthy donors. It is interesting to note that the frequencies of Th1 cells reactive to TAA-LPs were significantly increased after vaccination compared with before vaccination. In some patients, immune responses of TAA-LP-specific Th1 cells were remarkably augmented or elicited by repeated vaccination of TAA-SPs. Accumulating evidence suggests that TAA-LP-specific Th1 responses in cancer patients who received a CTL-epitope SP vaccination may be explained by intramolecular or intermolecular antigen spreading, which is known as a key phenomenon in successful cancer immunotherapy.50, 51
Conclusions and Future Directions
Genome-wide cDNA microarray analysis of gene expression profiles in comprehensive tumor types allowed us to identify various novel TAAs that show unique and ideal gene expression patterns, such as CTA and OFA. Recent studies showed the TAA-derived SPs and LPs can induce tumor-specific CTLs and Th1 cells in cancer patients' peripheral blood. A phase II study of TAA-SP vaccine immunotherapy induced improvements of progression-free survival and OS in advanced HNSCC patients. To improve a weak point of therapeutic SP vaccine, we have identified TAA-LPs encompassing CTL-epitopes. The TAA-LPs were highly immunogenic and had the capacity to induce not only Th1 cells but also CTLs through cross-presentation. These findings suggest that TAA-LPs may provide a synergistic effect on induction of tumor-specific CTLs in combination with CTL-epitope SP vaccine therapy and may improve clinical outcomes, as reported.52-57 Recent clinical trials have validated that blockades of PD-L1/PD-1 signaling and CTLA-4 is a meaningful immune therapeutic regimen and have shown durable antitumor responses. A combination therapy with immune checkpoint blockade and TAA-derived peptide vaccine therapy has been shown to have synergistic antitumor effects.2 These studies suggest that combination immunotherapy with TAA-LP and/or -SP vaccines and immune checkpoint blockade may be a good candidate for future cancer immunotherapy.
Acknowledgments
We thank Satoru Senju, Hirotake Tsukamoto, and Atsushi Irie for their cooperation. This work was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology (Kakenhi Grant No. 22133005), the Japan Society for Promotion of Science (Kakenhi Grant No. 24300334), and funding from OncoTherapy Science, Inc. (to Y.N.).
Disclosure Statement
Yasuharu Nishimura is supported by funding from OncoTherapy Science, Inc. The remainder of the authors have no conflict of interest.




