5-Lipoxygenase and cysteinyl leukotriene receptor 1 regulate epidermal growth factor-induced cell migration through Tiam1 upregulation and Rac1 activation
Abstract
Cell migration is an essential step for tumor metastasis. The small GTPase Rac1 plays an important role in cell migration. Previously, we reported that epidermal growth factor (EGF) induced two waves of Rac1 activation; namely, at 5 min and 12 h after stimulation. A second wave of EGF-induced Rac1 activation was required for EGF-induced cell migration, however, the spatiotemporal regulation of the second wave of EGF-induced Rac1 activation remains largely unclear. In this study, we found that 5-lipoxygenase (5-LOX) is activated in the process of EGF-induced cell migration, and that leukotriene C4 (LTC4) produced by 5-LOX mediated the second wave of Rac1 activation, as well as cell migration. Furthermore, these effects caused by LTC4 were found to be blocked in the presence of the antagonist of cysteinyl leukotriene receptor 1 (CysLT1). This blockage indicates that LTC4-mediated CysLT1 signaling regulates the second EGF-induced wave of Rac1 activation. We also found that 5-LOX inhibitors, CysLT1 antagonists and the knockdown of CysLT1 inhibited EGF-induced T cell lymphoma invasion and metastasis-inducing protein 1 (Tiam1) expression. Tiam1 expression is required for the second wave of EGF-induced Rac1 activation in A431 cells. Therefore, our results indicate that the 5-LOX/LTC4/CysLT1 signaling pathway regulates EGF-induced cell migration by increasing Tiam1 expression, leading to a second wave of Rac1 activation. Thus, CysLT1 may serve as a new molecular target for antimetastatic therapy. In addition, the CysLT1 antagonist, montelukast, which is used clinically for allergy treatment, might have great potential as a novel type of antimetastatic agent.
Cell migration is an essential feature of many physiological processes, including development, tissue remodeling and immune responses. It is also a required step in cancer metastasis. Cell migration is a multistep process that includes polarization, protrusion of the leading edge of the cell, formation of focal adhesion, translocation of the cell body, and retraction of the rear of the cell.1 When a cell migrates, the nucleation and polymerization of cytoskeletons drive the protrusion of the leading edge of the cell, particularly the lamellipodia and filopodia, a process that produces a locomotive force. The small GTPases of the Rho family, namely, Rac1 and Cdc42, are key molecules regulating morphological changes, as they regulate the formation of lamellipodia and filopodia, respectively.2-4 These Rho family small GTPases are largely known as binary switches that cycle between an active, GTP-bound, state and an inactive, GDP-bound, state. Guanine nucleotide exchange factor (GEF), which is activated by extracellular stimuli, catalyzes the exchange of GDP with GTP with small GTPases, resulting in the binding of small GTPases to effectors.5 However, very little is known about the specific role GEF play in regulating cell migration.
Previously, we reported that epidermal growth factor (EGF) induces biphasic lamellipodia formation, which was observed at 5 min and 12 h, in the process of cell migration of human epidermoid carcinoma A431 cells and human thyroid carcinoma TT cells. These two waves of lamellipodia formation were reflected by two waves of EGF-induced Rac1 activation, which were observed at 2–5 min and 6–12 h, respectively.6 The biphasic timing of Rac1 activation mirrors the biphasic formation of lamellipodia. The first wave of lamellipodia formation appears to be nonpolarized, but the second wave forms at the leading edge and is polarized. Because inhibition of the second wave of Rac1 activation is sufficient to inhibit cell migration, the second wave is required for EGF-induced cell migration. However, the mechanisms of regulation of this second wave of Rac1 activation are not fully understood.
5-Lipoxygenase (5-LOX) is an enzyme that catalyzes the conversion of arachidonate into the unstable epoxide intermediate leukotriene A4 (LTA4).7 When 5-LOX is activated by an extracellular stimulus, it translocates to the nuclear membrane, where it receives arachidonate donated by 5-LOX-activating protein, and catalyzes its conversion into LTA4.8 LTA4 is rapidly converted into either the leukotriene LTB4 by LTA4 hydrolase9 or LTC4 by LTC4 synthase.10 The latter is further converted into stable metabolites leukotrienes LTD4 and LTE4.11 LTB4 exert their effects by binding to their receptors, BLT1 and BLT2.12, 13 LTC4, LTD4 and LTE4 do the same by binding to their receptors, cysteinyl leukotriene receptors 1 and 2 (CysLT1 and CysLT2).14, 15 LTB4 is a potent chemoattractant for neutrophils,16 whereas LTC4, LTD4 and LTE4 activate maturation and migration of dendritic cells and astrocytes.17, 18 Previous reports imply that 5-LOX signaling is also involved in cancer development and progression. For example, the 5-LOX pathway facilitates the survival of prostate cancer cells and colon cancer cells,19, 20 as well as polymerization of actin in epithelial cancer cells.21 5-LOX inhibitors exhibit chemopreventive and chemotherapeutic properties by suppressing tumor growth in vivo and in vitro.22
In the present study, we found that the 5-LOX inhibitors AA-861 and BU-4664L suppress EGF-induced tumor cell migration by inhibiting the second wave of Rac1 activation, indicating that 5-LOX signaling is involved in this second wave. Moreover, we examined the role of 5-LOX signaling in the second EGF-induced wave of Rac1 activation.
Materials and Methods
Chemotaxis chamber assay
A431 cells suspended in DMEM supplemented with 0.2% calf serum (CS) were incubated in the upper chamber; the lower chamber contained DMEM supplemented with 0.2% CS with or without EGF (30 ng/mL). The drugs were added to both chambers. After 24 h incubation, the filter was fixed with methanol and stained with hematoxylin. The cells attached to the lower side of the filter were counted under the microscope.
Confocal laser scanning microscopy
Cells were fixed with 3% paraformaldehyde for 15 min and then permeabilized with 0.5% Triton X-100 in PBS for 5 min. After rinsing three times with PBS, the cells were incubated in blocking buffer (1% BSA in PBS) for 30 min and then stained with Texas Red-X phalloidin (1:100; Molecular Probes, Eugene, OR, USA) at room temperature for 1 h. Fluorescence images were obtained using a confocal laser scanning microscope system FV1000 (Olympus, Tokyo, Japan). Lamellipodia formation (%) is the proportion of cells with lamellipodia in the total cell count.
Detection of active Rac1
Rac1-GTP pull-down assay was carried out as described previously.6
Leukotriene C4 measurement
A431 cells were pretreated with or without drugs for 15 min and then stimulated with 30 ng/mL EGF. Following incubation, LTC4 was extracted by adding methanol into the culture medium. Insoluble matter was cleared by centrifugation at 10 000 g for 5 min. The supernatant was evaporated and reconstituted with assay buffer, and then LTC4 was purified through a Sep-Pak column (Waters Associates, Milford, MA, USA) and measured using a specific immunoassay (Cayman) according to the manufacturer's instructions.
siRNA transfection
siRNA double-stranded oligonucleotides designed to interfere with the expression of CysLT1 (sense 5′-UGUUUGUUGGCUUUAUCAUCCCUUU-3′, HSS116670 [Invitrogen, Carlsbad, CA, USA]) were used, and Stealth RNAi Negative Control (Invitrogen), was used as a negative control. Reverse transfection was demonstrated by using Lipofectamine RNAiMAX reagent (Invitrogen) according to the manufacturer's instructions. After being trypsinized, cells were resuspended in antibiotic-free medium, and then mixed with OPTI-MEM (Invitrogen) containing 50 nM siRNA and Lipofectamine RNAiMAX. After incubation for 20 min at room temperature, cells were diluted with cultured medium and seeded into a 100-mm dish. siRNA-transfected cells were reseeded into a six-well plate for the detection of Tiam1 protein, or a 150 mm dish for the detection of active Rac1 72 h after transfection. The silencing of CysLT1 was detected by measuring the expression of each protein just before drug treatment.
Real-time RT-PCR
Total RNA was extracted from A431 cells using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Total RNA (2 μg) was mixed with M-MLV reverse transcriptase (Promega, Madison, WI, USA) to produce complementary DNA. For real-time RT-PCR analysis, PCR mixtures were initially heated at 95°C for 10 s, and then at 95°C for 3 s, 61°C for 10 s and 72°C for 15 s for 50 cycles. Ribosomal protein L37a (RPL37A) was used an endogenous control, as reported previously.23 The primers designed for quantitative real-time RT-PCR analysis were as follows: Tiam1 sense 5′-TGAGATCTGACTGCGTCACC-3′ and antisense 5′-GGCTTCAGAACCAAGTCAGC-3′; RPL37A sense 5′-ATTGAAATCAGCCAGCACGC-3′ and antisense 5′-GCAGGAACCACAGTGCCAGATCC-3′. Results are described as the Tiam1/RPL37A ratio.
Statistical analysis
All statistical analyses in bar plots were performed with a two-tailed paired Student's t-test.
Other experimental procedures are outlined in the Supporting Information.
Results
5-lipoxygenase inhibitors inhibit the second epidermal growth factor-induced wave of lamellipodia formation
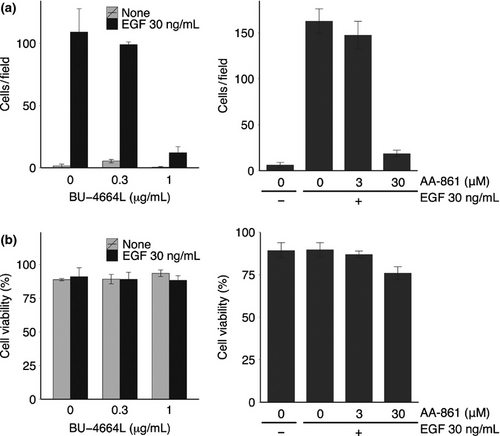
We first investigated the effect of 5-LOX inhibitors on cell migration following cytoskeletal remodeling. Earlier reports show that EGF-induced actin remodeling is regulated by 5-LOX and its products in epidermoid carcinoma A431cells.21 As shown in Figure 1, BU-4664L24, 25 and AA-861 inhibited EGF-induced cell migration of A431 cells at IC50 values of 0.66 μg/mL and 9.0 μM, respectively, without affecting cell viability. We previously found that EGF induced two waves of lamellipodia formation, at 5 min and 12 h after stimulation;6 therefore, we evaluated the effect of these 5-LOX inhibitors on each wave of lamellipodia formation. We found that BU-4664L and AA-861 did not inhibit the first wave of lamellipodia formation (Fig. 2a), but they inhibited the second wave at IC50 values of 0.69 μg/mL and 11.5 μM, respectively (Fig. 2b). These results indicate that 5-LOX played an important role in the second EGF-induced wave of lamellipodia formation in A431 cells.
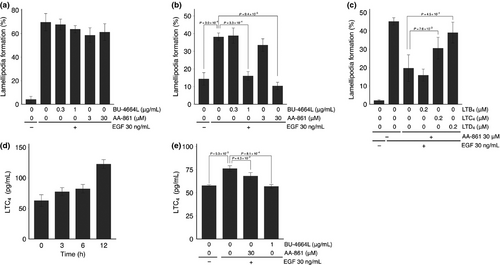
Because 5-LOX catalyzes the formation of LTB4, LTC4, LTD4 and LTE4, we then examined which leukotrienes were involved in the second EGF-induced wave of lamellipodia formation. The inhibition of this second wave by 5-LOX inhibitors was eliminated by the addition of cysteinyl leukotrienes LTC4 and LTD4, but not LTB4 (Fig. 2c). Furthermore, the total amount of LTC4 in cells and in culture medium increased for up to 12 h, and the 5-LOX inhibitor suppressed the amount of LTC4 (Fig. 2d,e). These results indicate that cysteinyl leukotrienes such as LTC4 and LTD4 produced by EGF stimulation mediated the second EGF-induced wave of lamellipodia formation in A431 cells.
5-lipoxygenase inhibitors inhibit the second epidermal growth factor-induced wave of Rac1 activation
Previously we reported that EGF induced distinct waves of Rac1 activation, and that the second wave Rac1 activation regulated the second wave of lamellipodia formation.6 Because 5-LOX inhibitors inhibited this second wave, we next examined the effect of 5-LOX inhibitors on the second wave of Rac1 activation. As shown in Figure 3(a,b), both BU-4664L and AA-861 inhibited the second wave at a dose range similar to that that inhibited lamellipodia formation (Fig. 3b). In contrast, both inhibitors failed to inhibit the first wave of Rac1 activation 2 min after EGF stimulation (Fig. 3a). These results suggest that the second EGF-induced wave of Rac1 activation was mediated by LTC4 and LTD4 produced by 5-LOX in A431 cells.

Involvement of cysteinyl leukotriene receptor 1 signaling in the second epidermal growth factor-induced wave of Rac1 activation
Next, we examined the LTC4-mediated signaling in the second wave of Rac1 activation. CysLT1 and CysLT2 have been identified as receptors for LTC4.14, 15 When A431 cells were stimulated with EGF, the expression levels of CysLT2 were not affected by EGF stimulation, whereas those of CysLT1 increased at 6 h (Fig. S1). We found that the CysLT1-specific antagonist, MK-571 or montelukast, inhibited EGF-induced cell migration in A431 cells (Fig. S2). Therefore, the involvement of CysLT1 signaling in the second wave of Rac1 activation was examined. As shown in Figure 3(c,d), MK-571 and montelukast did not inhibit the first wave of Rac1 activation, but inhibited the second EGF-induced wave. These results indicate that LTC4-mediated CysLT1 signaling might regulate the second EGF-induced wave of Rac1 activation.
5-lipoxygenase/leukotriene C4/cysteinyl leukotriene receptor 1 signaling modulates the second epidermal growth factor-induced wave of Rac1 activation through upregulation of expression of T-cell lymphoma invasion and metastasis-inducing protein 1
We previously demonstrated that Tiam1 might function as the RacGEF responsible for the second EGF-induced wave of Rac1 activation by interacting with 14-3-3ζ. As shown in Figure 4(a) and in our previous report,6 the intracellular expression levels of Tiam1 were quite low in unstimulated A431 cells, but gradually increased within 9 h after EGF stimulation. Furthermore, an elevated level of the Tiam1 mRNA was detected by real time RT-PCR analysis in EGF-stimulated A431 cells (Fig. 4b); this observation suggests that Tiam1 expression was increased at the level of transcription. However, the regulatory mechanisms underlying EGF-increased Tiam1 expression have not yet been discovered. Therefore, we examined the possibility that CysLT1 signaling regulates the expression level of Tiam1. As shown in Figure 4(c,d), the CysLT1 antagonists MK-571 and montelukast suppressed the EGF-induced increase in Tiam1 expression at both the mRNA and protein levels. Similar results were obtained when TT cells (a human thyroid cancer cell line) or EC109 cells (a human esophageal cancer cell line), were used in place of A431 cells (Fig. S3). The 5-LOX inhibitors BU-4664L and AA-861 also inhibited expression, and addition of LTD4 nullified this inhibition (Figs 4e,S4). Taken together, 5-LOX/LTC4(D4)/CysLT1 signaling regulates the second EGF-induced wave of Rac1 activation and lamellipodia formation by modulating the expression level of Tiam1 protein.

Knockdown of cysteinyl leukotriene receptor 1 blocks epidermal growth factor-induced T-cell lymphoma invasion and metastasis-inducing protein 1 expression
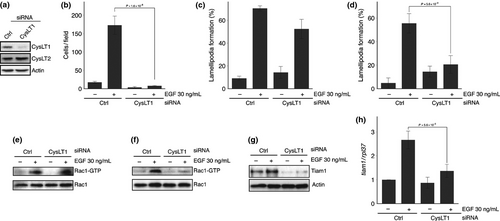
Finally, we confirmed the involvement of CysLT1 signaling in EGF-induced Tiam1 expression by using RNAi experiments. The successful knockdown of CysLT1 by siRNA was confirmed by western blotting (Fig. 5a). Silencing of CysLT1 expression consequently suppressed not only EGF-induced Tiam1 expression at both mRNA and protein levels, but also the second EGF-induced wave of Rac1 activation, lamellipodia formation and cell migration (Fig. 5b,d,f–h). In contrast, silencing of CysLT1 had little effect on the first EGF-induced wave of Rac1 activation and the corresponding lamellipodia formation (Fig. 5c,e). These results indicate that 5-LOX/LTC4(D4)/CysLT1 signaling regulated EGF-induced expression of Tiam1, resulting in the second EGF-induced wave of Rac1 activation and cell migration.
Discussion
In the present study, we investigated the involvement of 5-LOX and CysLT1 signaling in the process of EGF-induced cell migration by using small molecule inhibitors. Although EGF was found to induce biphasic lamellipodia formation and Rac1 activation in the process of cell migration, 5-LOX inhibitors were observed to selectively suppress the second EGF-induced wave of lamellipodia formation and Rac1 activation, as well as cell migration (Fig. 1a). Addition of LTC4 or LTD4, but not LTB4, cancelled the inhibition by 5-LOX inhibitors, indicating that cysteinyl leukotrienes (LTC4/D4/E4) produced by 5-LOX mediate the second wave of lamellipodia formation and Rac1 activation as a mechanism of EGF-induced cell migration. We confirmed that EGF induced the increase in LTC4 production, as previously reported (Fig. 2d).26
Although cysteinyl leukotrienes (LTC4/D4/E4) are known to mediate their effects by binding to two different G protein-coupled receptors (CysLT1 and CysLT2), we demonstrated that the receptor subtype that mediated the effect of EGF was CysLT1. This conclusion is derived from the following findings: (i) the CysLT1-specific antagonists MK-571 and montelukast inhibited EGF-induced cell migration in A431 cells (Fig. S2); (ii) MK-571 and montelukast inhibited the second EGF-induced wave of Rac1 activation, but not the first wave of Rac1 activation (Fig. 3c,d); and (iii) the silencing of CysLT1 expression inhibited the second EGF-induced wave of Rac1 activation and lamellipodia formation, as well as migration (Fig. 5b,d,f–h). The involvement of cysteinyl leukotriene-mediated CysLT1 signaling in the migration of several types of cells is also reported.27-34 However, in contrast to these reports, cysteinyl leukotrienes did not induce cell migration in A431 cells alone, possibly because of their lower expression levels of CysLT1 (Figs S1,S5). Therefore, cysteinyl leukotriene-induced cell migration of A431 cells required the EGF signal-mediated upregulation of CysLT1. Indeed, cysteinyl leukotriene alone could induce cell migration only when A431 cells were stimulated with EGF in the presence of 5-LOX inhibitors (Fig. S6). Thus, EGF signaling is responsible not only for cysteinyl leukotriene production, but also for the upregulation of CysLT1 in cell migration. Similar observations that production of cysteinyl leukotriene and increased CysLT1 expression are required for astrocyte migration induced by transforming growth factor-β have been reported.18
The present study also demonstrates how CysLT1 signaling contributes to cell migration. Rac has been implicated in the regulation of the migration of various cell types. We previously found that the EGF-induced biphasic activation of Rac1 and the second-wave Rac1 activation require the upregulation of Tiam1, a member of the Rac GEF family.6 We also demonstrated that CysLT1 signaling played an important role in Tiam1 upregulation required for the second EGF-induced wave of Rac1 activation. This conclusion is supported by our findings that not only 5-LOX inhibitors but also CysLT1 antagonists inhibited the EGF-induced increase of Tiam1 expression, as well as the second EGF-induced wave of Rac1 activation (Figs 4a,b and 5). CysLT1-mediated Rac activation was also reported in other types of cells,31 but this activation was demonstrated to be dependent upon the time of LTD4 stimulation; maximum activation is reached after 15 min, and is mediated through tyrosine phosphorylation and activation of another Rac GEF, Vav2. Thus, our study is the first to demonstrate the involvement of CysLT1 signaling in the mechanism of the second wave of EGF-induced Rac1 activation. Conversely, EGF-induced leukotriene synthesis is reported to be mediated by Rac.35 Therefore, the first EGF-induced wave of Rac1 activation may be responsible for cysteinyl leukotriene production. However, we cannot yet exclude the possibility that the first EGF-induced wave of Rac1 activation is also involved in the mechanism underlying EGF-induced upregulation of CysLT1.
What has remained unclear is the role of CysLT2 in the process of cancer cell migration. As shown in Figure S1, CysLT2 could be detected in A431 cells, and its expression levels were not affected even when cells were stimulated with EGF. Nevertheless, LTC4 alone did not induce cell migration in A431 cells (Figs S1,S5). These results can be explained by other findings that CysLT2 has inhibitory effects against tumor progression and the migration of cancer cells;36, 37 therefore, LTC4 may bind to CysLT2, and the downstream signaling of CysLT2 could suppress cell migration when A431 cells are not stimulated with EGF. However, once cells are stimulated with EGF, the expression of CysLT1 is upregulated and LTC4 can bind and activate CysLT1, thereby generating signals leading to cell migration.
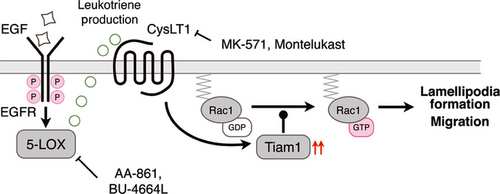
We suggest a model for the mechanism regulating the second EGF-induced wave of Rac1 activation in the process of cell migration, as shown in Figure 6. Our findings indicate that crosstalk between EGFR signaling and CysLT1 signaling is essential for epithelial cancer migration and invasion. This hypothesis is consistent with our previous chemical genomic study, which predicted that CysLT1 signaling might be closely related to Taim1-mediated Rac1 activation.38 Thus, CysLT1 is a core element of the regulating mechanism of cell migration and the second wave of Rac1 activation in our model. This may serve as a new molecular target for antimetastatic therapy. Because montelukast is already put on the market as a clinical drug for allergies, it also has great potential as an antimetastatic agent against a subset of cancer cells.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas and JSPS Fellows from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Disclosure Statement
The authors have no conflict of interest.




