Bortezomib therapy-related lung disease in Japanese patients with multiple myeloma: Incidence, mortality and clinical characterization
Funding information:
Janssen Pharmaceutical K.K.
Abstract
Because of the potentially high mortality rate (6.5%) associated with bortezomib-induced lung disease (BILD) in Japanese patients with relapsed or refractory multiple myeloma, we evaluated the incidence, mortality and clinical features of BILD in a Japanese population. This study was conducted under the Risk Minimization Action Plan (RMAP), which was collaboratively developed by the pharmaceutical industry and public health authority. The RMAP consisted of an intensive dissemination of risk information and a recommended countermeasure to health-care professionals. All patients treated with bortezomib were consecutively registered in the study within 1 year and monitored for emerging BILD. Of the 1010 patients registered, 45 (4.5%) developed BILD, 5 (0.50%) of whom had fatal cases. The median time to BILD onset from the first bortezomib dose was 14.5 days, and most of the patients responded well to corticosteroid therapy. A retrospective review by the Lung Injury Medical Expert Panel revealed that the types with capillary leak syndrome and hypoxia without infiltrative shadows were uniquely and frequently observed in patients with BILD compared with those with conditions associated with other molecular-targeted anticancer drugs. The incidence rate of BILD in Japan remains high compared with that reported in other countries, but the incidence and mortality rates are lower than expected before the introduction of bortezomib in Japan. This study describes the radiographic pattern and clinical characterization of BILD in the Japanese population. The RMAP seemed clinically effective in minimizing the BILD risk among our Japanese population.
Bortezomib (Velcade®) is a first-in-class proteasome inhibitor that selectively and reversibly blocks the 26S chymotrypsin-like activity of the proteasome and induces functional derangement in malignant cells that lead to apoptosis.1 Bortezomib is currently used as the primary treatment for multiple myeloma (MM) and lymphoma worldwide. It was approved in Japan in December 2006 by the Ministry of Health, Labor, and Welfare (MHLW) for the treatment of relapsed and refractory MM based on the Phase III APEX trial (n = 669) data.2 This study did not include Japanese patients, whereas the single-arm Phase II clinical trial3 (n = 33) was conducted with only Japanese patients. Before bortezomib approval in Japan, 4 (30.7%) of 13 patients with myeloma who were treated with privately imported bortezomib experienced fatal pulmonary complications.4 Of these four patients, 2 (15.4%) died from respiratory failure. In addition, one case (3.3%) of fatal interstitial lung disease (ILD) was reported in the Japanese Phase II clinical trial.3
These reports raised huge safety concerns with the Japanese Society of Hematology (JSH), the MHLW and the Pharmaceutical and Medical Device Agency (PMDA) regarding bortezomib treatment, leading the JSH to conduct an urgent questionnaire-based survey. The results of this survey indicated that 7 (15.2%) of 46 patients had bortezomib therapy-related lung injuries, with three deaths (6.5%).5 Concern about bortezomib-induced lung disease (BILD) was aroused shortly after fatal gefitinib-induced acute ILD became an issue in the postmarket stage in Japan around 2003. As a result, the postmarket risk management policy developed by the public health-care authority in Japan became stricter for new drugs, especially for the risk management of fatal drug-induced lung diseases. Because of this environment, BILD was identified as an important risk of bortezomib therapy for relapsed and refractory MM in the Japanese population. This identification led to the design and development of the Risk Minimization Action Plan (RMAP) for fatal BILD in collaboration with the pharmaceutical industry and the PMDA.6 In this study, we evaluated the incidence, mortality and clinical features of BILD in the Japanese population by using data from the patient registry study under the enforcement of the RMAP and investigated the effect of the RMAP for event rate estimation.
Materials and Methods
Patient registration
This registry study was conducted after bortezomib was launched in accordance with Good Postmarketing Surveillance Practice and authorized by the MHLW in Japan. All the patients with relapsed and refractory MM treated with bortezomib were consecutively registered and enrolled in this study between 1 December 2006 and 30 November 2007. The patients were monitored up to bortezomib treatment termination or 3 years from the first bortezomib dose.
Risk minimization action plan
- Patients who had poor Eastern Cooperative Oncology Group performance status (ECOG PS < 3) should be excluded.
- Preexisting lung disorder by radiological evaluation should be excluded.
- Routine monitoring of pulmonary function with computed tomography as needed, chest radiography and SpO2 measurement for early detection of an event should be performed.
- ILD biomarker (KL-6, SP-A, and SP-D) test before/after bortezomib treatment is recommended.
- Clinical tests for differential diagnosis and treatment for the emerging event are also recommended.
In addition, Janssen Pharmaceutical K.K. provided radiographic and clinical consultation to the Lung Injury Medical Expert Panel members to eliminate the risk of a preexisting pulmonary comorbidity. The RMAP has been enforced since immediately after the introduction of bortezomib in the Japanese market.
Collected data
Demographic and baseline data were collected for each patient, including sex, age, height, weight, type and clinical stage of MM, ECOG PS, comorbidity, history of smoking and abnormality on chest radiography for the purpose of post-hoc analysis of risk factors for BILD. The case report form intensively focused on the bortezomib dose and administration schedule, as well as treatment information, such as concomitant drug usage to evaluate causality of events. Once a pulmonary complication was reported, all clinical course information related to the event and clinical data (e.g. complete blood count, biochemical test, ILD biomarker, SpO2 and radiographic data) was collected in a post-hoc manner to verify the primary pulmonary complication and categorization of clinical features.
Lung injury medical expert panel
The Lung Injury Medical Expert Panel was organized with the aims of verifying the causal relationship between the events and bortezomib treatment, and evaluating the clinical features associated with the radiographic pattern of BILD. This panel consisted of pulmonologists, radiologists, cardiologists, hematologists, oncologists and pathologists selected from those who were committee members of other drug-induced pulmonary complication committees, such as those for gefitinib.
Case definition and review algorithm
Pulmonary-related adverse events were automatically retrieved on the basis of preferred terms from the Medical Dictionary for Regulatory Activities Japanese version 11.0 (Regulatory Science of Japan, Tokyo, Japan). An event with positive causality judged by the physician was defined as physician-reported BILD. The physician-reported BILD was further evaluated by the Lung Injury Medical Expert Panel for the purpose of causality verification and categorization of pulmonary complication types, radiologic patterns and event outcomes. The panel defined each case as BILD based on exclusive diagnosis. The major causality review points considered were as follows: non-infection related, noncardiac related, improvement or disappearance of symptoms by bortezomib discontinuation, and reproducible events.
Statistical analysis
The expected mortality rate of physician-reported BILD in the Japanese population was set at 6.5%, as reported by the JSH,5 and the minimum sample size was set at 500 patients to produce a two-sided 95% confidence interval (CI) with a width equal to 4.5%. Under these circumstances, if 44 of 500 patients developed fatal BILD, the estimated lower limit of the 95% CI would exceed 6.5%. In this case, the potential risk of fatal BILD would be >6.5% in the Japanese population.
As a primary analysis, physician-reported BILD was summarized into frequency and rate (%) with an estimated 95% CI. Continuous variables were summarized into the mean and standard deviation or median with range, and categorical variables were summarized into frequency and rate. All statistical analyses were performed using the sas software package (version 9.2; SAS Institute, Cary, NC, USA).
Ethical considerations
Before collecting data from the patients who were treated with bortezomib, the privacy policy and possible disclosure of relevant data under anonymous filtering were explained to the patients, and their written consent was obtained.
Results
Patient demographics
Fixed data from 1010 patients registered at 279 institutions were obtained for this survey. Demographic data are shown in Table 1. The median age of the patients was 64 years (range, 31–92 years), with women representing 46.6% of the population. Most of the patients (97.7%) had a good ECOG PS (PS 0, 35.9%; PS 1, 40.5%; PS 2, 21.3%) and 26.7% had a history of smoking. The comorbidities observed at baseline were cardiac disease (10.4%), lung disease (9.6%), liver dysfunction (5.1%) and renal dysfunction (25.0%). Concurrent use of corticosteroids with bortezomib treatment was observed in 85% of the patients.
| Characteristics | All (n = 1010) | |
|---|---|---|
| n | % | |
| Sex | ||
| Male | 541 | 53.6 |
| Female | 469 | 46.4 |
| Age, years Median = 64 (range, 31–92) | ||
| 30–39 | 9 | 0.9 |
| 40–49 | 67 | 6.6 |
| 50–59 | 240 | 23.8 |
| 60–69 | 425 | 42.1 |
| 70–80 | 236 | 23.4 |
| 80–90 | 31 | 3.1 |
| ≥90 | 1 | 0.1 |
| ND | 1 | 0.1 |
| History of smoking | ||
| No | 647 | 64.1 |
| Yes | 270 | 26.7 |
| ND | 93 | 9.2 |
| Performance status at baseline | ||
| 0 | 363 | 35.9 |
| 1 | 409 | 40.5 |
| 2 | 215 | 21.3 |
| 3 | 18 | 1.8 |
| 4 | 5 | 0.5 |
| Comorbidity of lung disease | ||
| No | 913 | 90.4 |
| Yes | 97 | 9.6 |
| Comorbidity of cardiac disease | ||
| No | 905 | 89.6 |
| Yes | 105 | 10.4 |
| Liver dysfunction at baseline | ||
| No | 958 | 94.9 |
| Yes | 51 | 5.1 |
| ND | 1 | 0.1 |
| Renal dysfunction at baseline | ||
| No | 757 | 75.0 |
| Yes | 252 | 25.0 |
| ND | 1 | 0.1 |
| Concurrent use of steroids as part of multiple myeloma regimen | ||
| No | 152 | 15.1 |
| Yes | 858 | 85.0 |
- ND, no data.
Incidence, outcome and time to onset
Pulmonary-related adverse events were observed in 160 patients (15.8%; 95% CI, 13.7–18.2%). Physician-reported BILD was reported in 45 patients (4.5%; 95% CI, 3.3–5.9%). A total of 17 deaths were attributed to pulmonary-related adverse events. Of these deaths, 12 were caused by non-bortezomib-induced pulmonary-related (non-BILD) events (1.2%; 95% CI, 0.7–2.1%) and five deaths were due to physician-reported BILD (0.5%; 95% CI, 0.2–1.2%; Table 2). The mortality rate among physician-reported BILD was 11.1% (95% CI, 4.2–22.1%), which was similar to the mortality of non-BILD events at 10.4% (95% CI, 6.1–17.4%). The median times to onset for the physician-reported BILD and non-BILD were 14.5 (range, 1–83 days) and 28.5 days (range, 1–1117 days) from the first bortezomib dose, respectively. In addition, the median times to death from the onset for physician-reported BILD and non-BILD were 4.0 (range, 2–12 days) and 5.5 days (range, 1–312 days), respectively.
| All the patients (n = 1010) | Number of cases (% with 95% CI) | Number of deaths (% with 95% CI) | Mortality (% with 95% CI) |
|---|---|---|---|
| Reported pulmonary-related adverse events | 160 (15.8%, 13.7–18.2) | 17 (1.7%, 1.1–2.7) | 17/160 (10.6%, 6.7–16.4) |
| Reported pulmonary-related adverse events other than bortezomib-induced lung disease (non-BILD) | 115 (11.4%, 8.3–12.0) | 12 (1.2%, 0.7–2.1) | 12/115 (10.4%, 6.1–17.4) |
| Physician-reported bortezomib-induced lung disease (BILD) | 45 (4.5%, 3.3–5.9) | 5 (0.5%, 0.2–1.2) | 5/45 (11.1%, 4.2–22.1) |
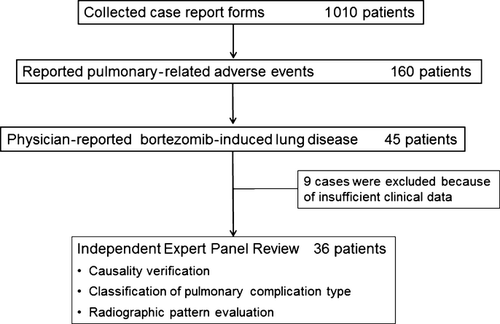
Causality verification and pulmonary event classification with radiographic pattern
In total, nine of the 45 patients with physician-reported BILD were excluded because of insufficient data, and the remaining 36 patients were evaluated for causality by the Lung Injury Medical Expert Panel (Fig. 1). In 26 of these patients, positive causality between bortezomib use and BILD was noted. Thus, the estimated concordance rate of causality between physician-reported BILD and that defined by the panel was 72.2% (95% CI, 56.0–84.2%). Most events in the remaining 10 patients appeared to be the consequence of heart failure and/or infections: heart failure (n = 1); heart failure complicated with pneumonia (n = 3); pneumonia (n = 3); bronchitis (n = 2); and transient hypoxia caused by loxoprofen sodium hydrate (n = 1).
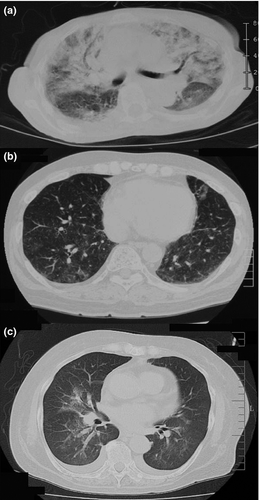
In the 26 patients with positive causality between bortezomib use and BILD, pulmonary events were classified by their radiological pattern, excluding that in one patient who had insufficient clinical data for evaluation. Based on the clinical course and radiographic data, the panel classified these patients into three major categories: interstitial pneumonia (n = 18); vascular hyperpermeability and capillary leak syndrome (CLS)-like (CLS-like type; n = 3); and hypoxia without significant radiological abnormality (hypoxia type; n = 4). Concerning the radiographic pattern, interstitial pneumonia was further categorized into three subtypes: diffuse alveolar damage (DAD; n = 3); hypersensitivity pneumonitis (HP; n = 8); and non-DAD/non-HP types (n = 7, Table 3). The typical radiographic images of each type are shown in Figure 2. DAD-type interstitial pneumonia exhibited patchy and diffuse shadows in both lungs and typical ground-glass opacities accompanying structural distortion (Fig. 2a). HP-type interstitial pneumonia displayed diffuse spotted ground-glass opacity in both lungs without volume loss or contraction bronchiectasis (Fig. 2b). Light ground-glass opacities near the pulmonary artery and substantial enlargement of vessel diameters, along with bronchia hypertrophy, were typically observed in CLS-like type (Fig. 2c).
| BILD | Radiologic patterns | Outcomes | Definition | ||
|---|---|---|---|---|---|
| Recovered/improved | Recovered not confirmed | Death | |||
| Interstitial pneumonian = 18 | Diffuse alveolar damage type (DAD)n = 3 | 1 | — | 2 | Patchy or diffuse distribution of infiltration or ground-glass opacities accompanying structural distortion (e.g. contraction bronchiectasis) |
| Hypersensitivity pneumonitis type (HP)n = 8 | 7 | 1 | — | Faint and homogeneous ground-glass opacities with neither volume loss nor contraction bronchiectasis | |
| Non-DAD non-HP typen = 7 | 6 | 1 | — | Presence of abnormal shadows different from DAD or HP patterns | |
| Vascular hyperpermeabilityn = 3 | Capillary leak syndrome (CLS)-liken = 3 | 3 | — | — | Thickening of the interstitium or bronchial wall and narrowing of bronchial lumens with frequent pleural or cardiac effusion without evidence of concurrent heart failure |
| Hypoxia without significant radiological findingsn = 4 | 4 | — | — | Significant decrease in the saturation of oxygen without obvious radiological abnormalities | |
| Total: 25 patients | 21 | 2 | 2 | ||
Of the five fatal cases evaluated by the panel, two were defined as DAD type, and the other three were classified as pneumonia, heart failure complicated with pneumonia, and BILD with infection. Although the last patient exhibited an “interstitial pneumonia shadow,” it could not be categorized into a particular subtype because of insufficient radiographic data. The two patients with DAD-type interstitial pneumonia died despite corticosteroid pulse therapy.
Clinical characterization of bortezomib-induced lung disease
Interstitial pneumonia including DAD and HP types are common types of drug-induced lung disease. In this study, 18 of 26 cases were categorized as interstitial pneumonia; however, the CLS-like type and hypoxia-type were rare. All the patients with CLS-like type developed fever, hypoxia, and elevated C-reactive protein (CRP) and lactate dehydrogenase (LDH) levels, with no evidence of left atrial hypertension/congestive heart failure. In addition, all the patients with hypoxia type exhibited fever and elevated CRP and LDH levels. One of the patients with hypoxia-type reproducibly demonstrated marked hypoxia without significant radiological abnormality after bortezomib re-administration. The median time of emerging DAD-type, HP type, CLS-like type and hypoxia-type from the first bortezomib dose were 8, 15 and 14 days, respectively. The deviation of the upper limit of ILD biomarkers such as KL-6, SP-A and SP-D were observed in 58% of patients when BILD was emerged. There were 67% in DAD-type, 85% in HP type and 100% in CLS-like type.
Of the 26 patients, 23 (88.5%) were treated with corticosteroids, two of whom died, and 16 patients were treated with methylprednisolone pulse therapy (1000 or 500 mg/day) with or without oxygen therapy. Of these 16, 15 patients responded well to the interventions and recovered, but one died because of BILD complicated by a fungal infection. All three patients with DAD-type received the steroid pulse therapy with oxygen treatment, the use of which was successful in one patient. All the patients with CLS-like or hypoxia-type were treated with corticosteroids and responded well to the treatment. None of the patients has been histologically diagnosed and only one patient was examined with bronchoalveolar lavage; therefore, we could not evaluate the relationship with their radiographic findings.
Discussion
In this registry study, the incidence rate of physician-reported BILD was estimated to be 4.5% (95% CI, 3.3–5.9%) and that among patients who died was 0.5% (95% CI, 0.2–1.2%) based on the 1010 patients followed up for more than 3 years under implementation of the RMAP. This incidence was lower than our expected incidence rate of 15.2%, including a rate of 6.5% among patients who died. All the patients in this study were consecutively registered and continuously monitored in Japan from 1 December 2006 to 30 November 2007, providing a complete dataset to allow accurate estimation of incidence and mortality. The registry study design we used is potentially influenced by various biases and should be considered as a limitation in the interpretation of our results.
The most common form of drug-induced lung disease (DILD) is ILD; however, DILD involves several different tissues, including airways, lung parenchyma, mediastinum, pleura, pulmonary vasculature and/or the neuromuscular system.9 Gefitinib-related and erlotinib-related DILD are frequently of the DAD type, whereas the antiarrhythmic drug amiodarone, a bi-iodinated benzofuran derivative, induces a wide variety of DILD types, including bronchospasm, diffuse alveolar hemorrhage, lipoid pneumonia and drug-induced lupus.10 Investigators participating in the global phase III studies of temsirolimus and everolimus identified DILD in 2% and 14% of patients with advanced renal cell carcinoma, respectively, whereas in a retrospective, independent blind review, 29% and 39% of patients treated with temsirolimus and everolimus, respectively, had radiographic findings consistent with DILD.11, 12 These findings suggest that a wide variety of DILD types can be associated with each drug. In addition, asymptomatic DILD may cause underdiagnoses and an underestimation of incidence and mortality. As described by Gemma et al.,13 these limitations in the available data underline the difficulty in judging whether DILD is caused by the molecular-targeted anticancer agent. In this study, 20% of physician-reported BILD were excluded from the analysis due to the insufficient clinical data; this was one of the major limitations for estimation of true BILD and mortality rates.
The high incidence of physician-reported BILD in Japan compared with that in other countries is similar to that in reports on other DILD in a postmarketing surveillance (PMS) study setting in Japan. During the period in which the present study was conducted, the worldwide incidence of bortezomib therapy-related pulmonary disease was low at 0.08% (from 26 October 2006 to 25 October 2010; Johnson & Johnson Pharmaceutical [New Brunswick, NJ, USA] and Millennium Pharmaceutical [Cambridge, MA, USA]). Such differences between Japanese PMS data and data from other countries were also evident for gefitinib (5.8% vs 0.3%), erlotinib (4.5% vs 0.11%) and leflunomide (1.81% vs 0.017%).14-18
Possible explanations for these differences are potential ethnic or genetic differences between the Japanese population and the other populations and/or differences in the methods used in the studies. Horiuchi-Yamamoto et al.19 demonstrate a limitation in the comparative evaluation of different uncontrolled studies. Our study and another Japanese PMS study estimated the incidence of DILD based on patients that were consecutively registered and continuously monitored with a definite population and registration period, whereas the worldwide incidence of postmarket adverse events is estimated from either spontaneous reports or literature reports. Such data based on spontaneous reports without a large prospective study may lead to underdiagnoses and an underestimation of the incidence of DILD.9 In addition to the observed differences in DILD incidence, Azuma et al.18 report that acute exacerbations of idiopathic pulmonary fibrosis (IPF) are more frequent and severe in Japanese patients. A correlation between single nucleotide polymorphisms of surfactant protein D and interstitial pneumonia in the Japanese population is reported in Ishii et al.,20 suggesting that the Japanese might be more sensitive to DILD. A nationwide study designed to identify Japanese genetic variations related to acute exacerbations of IPF and DILD is now underway.
The characteristics of BILD in patients with MM include a relatively short median time to the onset of BILD and a variety of radiographic patterns, such as the DAD, HP, CLS-like and hypoxia types. In particular, two characteristics of BILD may explain why the mortality rate of this study was lower than expected: a lower incidence of DAD-type BILD and high concurrent corticosteroid use with bortezomib. DAD-type DILD is known as a poor prognostic indicator, and the mortality rate among patients who developed gefitinib-related DILD was as high as 30–40%, with all deaths associated with the DAD pattern.18 Similarly, the fatal case of BILD in the phase I/II study and two of the five fatal cases in this study were also of the DAD type. In contrast to the DAD type, the clinical characteristics of CLS-like and hypoxia-type BILD may respond better to corticosteroid therapy, lowering the mortality rate in these patients. For example, some investigators report that IL-6 and IFN-γ production cause pyrexia, and patients with severe hypoxia display high levels of CRP, IL-6 and TNF-α and elevated IL-6 mRNA expression level due to proteasome inhibition, suggesting that corticosteroid therapy would be an effective treatment for these types of BILD.21-24 Gotoh et al.5 report the incidence and mortality of BILD from the results of the JSH retrospective survey, and also suggest that the concomitant use of corticosteroids might reduce the risk of BILD as it shows an odds ratio of 0.055 (P = 0.024). In our study, 85% of patients concurrently received the corticosteroids as a part of the MM regimen, which might be one potential reason why our results for incidence and mortality were lower than expected. In addition, it should be noted that a report on the JSH retrospective survey encouraged the concurrent use of corticosteroids as a part of the MM regimen and resulted in a high concomitant use rate of the corticosteroids in our study.
Although the present study design provides accuracy in determining the population at risk and the best estimates of BILD incidence and mortality, several factors should be taken into consideration when interpreting the results. First, our study observed a 72.2% concordance rate between physician-reported BILD and the panel findings, suggesting that the incidence based on physician-reported BILD may be overestimated. Second, the RMAP is a strong bias to encourage physician report false-positive BILD, resulting in an increased incidence, and the practice of early intervention may lead to a decrease in mortality. Finally, it should be noted that if physicians do not know the specific clinical characteristics of DILD, then BILD may be underdiagnosed and, therefore, underestimated.
Since 2003 when gefitinib-induced DILD became a concern in Japan, most of the molecular targeted drugs have been investigated using all-case PMS. In addition, large-scale PMS data on DILD have been accumulated in Japan over the past decade. As Saito and Gemma25 report, these PMS data provide the characteristic features, incidence, mortality and risk factors of DILD. In addition, such accumulated information should ensure a favorable benefit–risk balance for drug usage with consideration of the DILD risk factors.
In conclusion, we observed lower incidence (4.5%) and mortality rates (0.5%) for BILD than expected in the Japanese patients with relapsed and refractory MM. These patients also exhibited unique clinical and radiographic features and a better prognosis than previously expected. Our findings suggest that a postmarketing noninterventional observational study design that incorporates consecutive registration and continuous monitoring of all patients under the RMAP will provide the most accurate results. This design is an effective way to manage the risk minimization and accurate estimation of the true incidence and mortality rates, and to identify clinical characteristics of pulmonary-related adverse events, especially for new molecular targeted anticancer drugs.
Acknowledgments
This study was sponsored by Janssen Pharmaceutical K.K. All of the data and results have been verified by the sponsor. We would like to acknowledge the patients who participated in this postmarketing surveillance study and the investigators and their staff, the members of the Velcade case review subcommittee (Hiroaki Arakawa, Atsuko Kurosaki, Yasuhito Terui, Yasunori Nakagawa, Hiroshi Homma, Masahiro Abe and Akinobu Yoshimura), and the medical advisors for the Velcade risk management plan (Kenshi Suzuki, Keiichi Nagao, and Sakae Homma).
Disclosure Statement
K. Yoshizawa, H. Y. Mukai, M. Miyazawa, M. Miyao, and Y. Ogawa are full-time employees of Janssen Pharmaceutical K.K. K. Ohyashiki received research grants from Janssen Pharmaceutical K.K. A. Gemma, K. Hatake, F. Sakai and S. Kudoh received consultant fees from Janssen Pharmaceutical K.K. All the remaining authors have no conflict of interest.




