Comparative study of the diversity of amino acids on human leucocyte antigen class II molecules in patients with acquired aplastic anaemia
Jun Qi
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorTianju Wang
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorManni Wang
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorPengcheng He
Department of Hematology, The First Affiliated Hospital of Xi'an Jiao Tong University, Xi'an, Shaanxi Province, China
Search for more papers by this authorYuhui Li
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorLixia Shang
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorLe Chen
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorXiaofang Wang
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorCorresponding Author
Hua Xu
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Correspondence
Chaofeng Ma and Hua Xu, HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Zhuque Road 407, Xi'an, Shaanxi Province 710061, China.
Email: [email protected] and [email protected]
Search for more papers by this authorCorresponding Author
Chaofeng Ma
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Correspondence
Chaofeng Ma and Hua Xu, HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Zhuque Road 407, Xi'an, Shaanxi Province 710061, China.
Email: [email protected] and [email protected]
Search for more papers by this authorJun Qi
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorTianju Wang
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorManni Wang
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorPengcheng He
Department of Hematology, The First Affiliated Hospital of Xi'an Jiao Tong University, Xi'an, Shaanxi Province, China
Search for more papers by this authorYuhui Li
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorLixia Shang
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorLe Chen
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorXiaofang Wang
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Search for more papers by this authorCorresponding Author
Hua Xu
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Correspondence
Chaofeng Ma and Hua Xu, HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Zhuque Road 407, Xi'an, Shaanxi Province 710061, China.
Email: [email protected] and [email protected]
Search for more papers by this authorCorresponding Author
Chaofeng Ma
HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Xi'an, Shaanxi Province, China
Correspondence
Chaofeng Ma and Hua Xu, HLA Laboratory, Shaanxi Province Blood Center, Institute of Xi'an Blood Bank, Zhuque Road 407, Xi'an, Shaanxi Province 710061, China.
Email: [email protected] and [email protected]
Search for more papers by this authorChaofeng Ma and Hua Xu contributed equally to this work.
Summary
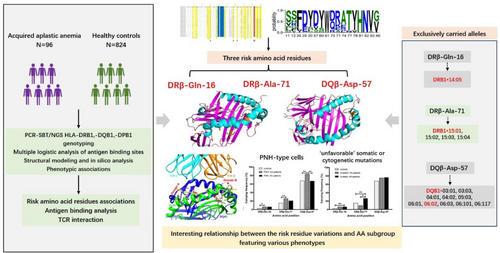
Human leucocyte antigen (HLA) class II molecules are critically involved in the pathology of acquired aplastic anaemia (AA) by regulating the immune response and autoreactive T cell-mediated haematopoietic cell death. In the study, amino acid residue variation and molecular structure of HLA class II have been initially investigated in 96 patients with AA. The frequencies of residues 9 and 57 in pocket 9 (P9) in DQB1, and amino acid positions 9, 11, 13, 16, 26, 38, 67 and 71 in the P4, P6 and P9 pockets in DRB1 were more prevalent among AA patients. By applying a multivariate recursive approach, the DRβ-Gln-16 (OR = 3.003, 95% CI = 1.468–6.145, pc = 0.003), DRβ-Ala-71 (OR = 1.924, 95% CI = 1.233–3.002, pc = 0.004) in P4/P7 and DQβ-Asp-57 (OR = 3.483, 95% CI = 1.079–11.242, pc = 0.037) in P9, these critical residues were significantly discovered as risk amino acid residues on the onset of AA, as well as associated with PNH-type cells and pathological somatic or cytogenetic mutations. In silico structural model analysis showed that identified DRβ-Ala-71 and DQβ-Asp-57 within the antigen-binding groove interacting with a more variable antigenic segments, may impact the repertoire of peptides presented, influence the interface HLA-antigen-T-cell receptor β (TCR β). These findings provided light on the immunogenetic pathophysiology of AA aetiology and their potential impact on upcoming immunotherapies.
Graphical Abstract
As an immunogenetic disease susceptibility factor, human leucocyte antigen (HLA) class II loci involve in the pathophysiology of acquired aplastic anaemia (AA) by regulating the immune response and autoreactive T-cell-mediated haematopoietic cell death. Distinctive amino acid configurations encoded by the at-risk HLA alleles enriched in AA patients impact the immune presentation of the peptide repertoire derived from haematopoietic stem/progenitor cells. DRβ-Ala-71, DQβ-Asp-57 and DRβ-Gln-16 are discovered as crucial risk amino acid residues in HLA II class proteins on the onset of AA, as well as associated with paroxysmal nocturnal haemoglobinuria (PNH)-type cells and pathological somatic or cytogenetic mutations. DRβ-Ala-71 and DQβ-Asp-57 within the antigen-binding groove interacting with a more variable antigenic segments, may impact the repertoire of peptides presented, influence the interface HLA-antigen-T-cell receptor β (TCR β).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
Supporting Information
| Filename | Description |
|---|---|
| bjh19899-sup-0001-TableS1.docxWord 2007 document , 21.7 KB |
Table S1. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Luzzatto L, Risitano AM. Advances in understanding the pathogenesis of acquired aplastic anaemia. Br J Haematol. 2018; 182(6): 758–776. https://doi.org/10.1111/bjh.15443
- 2Zhang XT, Zhang YN, Zhu JJ, Wang X, Cao J, Chen W, et al. The efficacy and safety of cyclosporine a plus androgen versus androgen alone for adult patients with non-severe aplastic anemia in China: a meta-analysis of randomized controlled trials. Hematology. 2022; 27: 733–741. https://doi.org/10.1080/16078454.2022.2081008
- 3Li H, Zhou C, Shen Y, Xu M, Wu D, Ye B. Research progress on the hematopoietic microenvironment in aplastic anemia. Eur J Haematol. 2023; 111(2): 172–180. https://doi.org/10.1111/ejh.13991
- 4Kikkawa E, Shiina T, Shigenari A, Ozaki Y, Suzuki S, Ando K, et al. Detection of 6pLOH in an aplastic anemia patient by in phase HLA genotyping. HLA. 2020; 95(5): 465–469. https://doi.org/10.1111/tan.13807
- 5Imi T, Katagiri T, Hosomichi K, Zaimoku Y, Hoang Nguyen V, Nakagawa N, et al. Sustained clonal hematopoiesis by HLA-lacking hematopoietic stem cells without driver mutations in aplastic anemia. Blood Adv. 2018; 2(9): 1000–1012. https://doi.org/10.1182/bloodadvances.2017013953
- 6Mizumaki H, Hosomichi K, Hosokawa K, Yoroidaka T, Imi T, Zaimoku Y, et al. A frequent nonsense mutation in exon 1 across certain HLA-A and -B alleles in leukocytes of patients with acquired aplastic anemia. Haematologica. 2021; 106(6): 1581–1590. https://doi.org/10.3324/haematol.2020.247809
- 7Chen B, Khodadoust MS, Olsson N, Wagar LE, Fast E, Liu CL, et al. Predicting HLA class II antigen presentation through integrated deep learning. Nat Biotechnol. 2019; 37(11): 1332–1343. https://doi.org/10.1038/s41587-019-0280-2
- 8Pagliuca S, Gurnari C, Rubio MT, Visconte V, Lenz TL. Individual HLA heterogeneity and its implications for cellular immune evasion in cancer and beyond. Front Immunol. 2022; 13:944872. https://doi.org/10.3389/fimmu.2022.944872
- 9Saunthararajah Y, Nakamura R, Nam JM, Robyn J, Loberiza F, Maciejewski JP, et al. HLA-DR15 (DR2) is overrepresented in myelodysplastic syndrome and aplastic anemia and predicts a response to immunosuppression in myelodysplastic syndrome. Blood. 2002; 100(5): 1570–1574.
- 10Liu S, Li Q, Zhang Y, Li Q, Ye B, Wu D, et al. Association of human leukocyte antigen DRB1*15 and DRB1*15:01 polymorphisms with response to immunosuppressive therapy in patients with aplastic anemia: a meta-analysis. PLoS One. 2016; 11:e0162382. https://doi.org/10.1371/journal.pone.0162382
- 11Liang L, Li N, Wang Y, Luo S, Song Y, Fang B. Human leukocyte antigen-DRB1 gene polymorphism and aplastic anemia: a meta-analysis. Medicine. 2023; 102(20):e33513. https://doi.org/10.1097/MD.0000000000033513
- 12Deng XZ, Du M, Peng J, Long JX, Zheng CJ, Tan Y, et al. Associations between the HLA-A/B/DRB1 polymorphisms and aplastic anemia: evidence from 17 case-control studies. Hematology. 2018; 23(3): 154–162. https://doi.org/10.1080/10245332.2017.1375064
- 13Camitta BM, Storeb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982; 306(11): 645–652. https://doi.org/10.1056/NEJM198203183061105
- 14Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014; 124(18): 2804–2811. https://doi.org/10.1182/blood-2014-02-522128
- 15Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016; 172(2): 187–207. https://doi.org/10.1111/bjh.13853
- 16Qi J, Wang TJ, Li HX, Wu D, du D, Wu JH, et al. Association of HLA class II (-DRB1, -DQB1, -DPB1) alleles and haplotypes on susceptibility to aplastic anemia in northern Chinese Han. Hum Immunol. 2020; 81(12): 685–691. https://doi.org/10.1016/j.humimm.2020.07.001
- 17 IUPAC-IUB Commission on Biochemical Nomenclature. Tentative rules. A one-letter notation for amino acid sequences. Biochim Biophys Acta. 1968; 168(1): 6–10. https://doi.org/10.1042/bj1130001
- 18Pierini F, Lenz TL. Divergent allele advantage at human MHC genes: signatures of past and ongoing selection. Mol Biol Evol. 2018; 35(9): 2145–2158. https://doi.org/10.1093/molbev/msy116
- 19Smith KJ, Pyrdol J, Gauthier L, Wiley DC, Wucherpfennig KW. Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med. 1998; 188(8): 1511–1520. https://doi.org/10.1084/jem.188.8.1511
- 20Siebold C, Hansen BE, Wyer JR, Harlos K, Esnouf RE, Svejgaard A, et al. Crystal structure of HLA-DQ0602 that protects against type 1 diabetes and confers strong susceptibility to narcolepsy. Proc Natl Acad Sci USA. 2004; 101(7): 1999–2004. https://doi.org/10.1073/pnas.0308458100
- 21Jiang W, Birtley JR, Hung SC, Wang W, Chiou SH, Macaubas C, et al. In vivo clonal expansion and phenotypes of hypocretin-specific CD4+ T cells in narcolepsy patients and controls. Nat Commun. 2019; 10(1): 5247. https://doi.org/10.1038/s41467-019-13234-x
- 22Lang HL, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002; 3(10): 940–943. https://doi.org/10.1038/ni835
- 23Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal omega. Mol Syst Biol. 2011; 7: 539. https://doi.org/10.1038/msb.2011.75
- 24Kovalchik KA, Ma Q, Wessling L, Saab F, Duquette JD, Kubiniok P, et al. MhcVizPipe: a quality control software for rapid assessment of small- to large-scale immunopeptidome datasets. Mol Cell Proteomics. 2022; 21(1):100178. https://doi.org/10.1016/j.mcpro.2021.100178
- 25Yin Y, Wang XX, Mariuzza RA. Crystal structure of a complete ternary complex of T-cell receptor, peptide-MHC, and CD4. Proc Natl Acad Sci USA. 2012; 109(14): 5405–5410. https://doi.org/10.1073/pnas.1118801109
- 26Sethi DK, Gordo S, Schubert DA, Wucherpfennig KW. Crossreactivity of a human autoimmune TCR is dominated by a single TCR loop. Nat Commun. 2013; 4: 2623. https://doi.org/10.1038/ncomms3623
- 27Zeng W, Maciejewski JP, Chen G, Young NS. Limited heterogeneity of T cell receptor BV usage in aplastic anemia. J Clin Invest. 2001; 108(5): 765–773. https://doi.org/10.1172/JCI12687
- 28Frommer L, Flesch BK, König J, Kahaly GJ. Amino acid polymorphisms in Hla class II differentiate between thyroid and polyglandular autoimmunity. J Clin Endocrinol Metab. 2020; 105(6): 1737–1747. https://doi.org/10.1210/clinem/dgz164
10.1210/clinem/dgz164 Google Scholar
- 29Hollenbach JA, Norman PJ, Creary LE, Damotte V, Montero-Martin G, Caillier S, et al. A specific amino acid motif of HLA-DRB1 mediates risk and interacts with smoking history in Parkinson's disease. Proc Natl Acad Sci USA. 2019; 116(15): 7419–7424. https://doi.org/10.1073/pnas.1821778116
- 30Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, Jia X, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet. 2012; 44(3): 291–296. https://doi.org/10.1038/ng.1076
- 31Saeki H, Kuwata S, Nakagawa H, Etoh T, Yanagisawa M, Miyamoto M, et al. Analysis of disease-associated amino acid epitopes on HLA class II molecules in atopic dermatitis. J Allergy Clin Immunol. 1995; 96(6 Pt 2): 1061–1068. https://doi.org/10.1016/s0091-6749(95)70191-5
- 32Ling SF, Viatte S, Lunt M, van Sijl AM, Silva-Fernandez L, Symmons DPM, et al. HLA-DRB1 amino acid positions 11/13, 71, and 74 are associated with inflammation level, disease activity, and the health assessment questionnaire score in patients with inflammatory polyarthritis. Arthritis Rheumatol. 2016; 68(11): 2618–2628. https://doi.org/10.1002/art.39780
- 33Zhao LP, Papadopoulos GK, Moustakas AK, Bondinas GP, Carlsson A, Larsson HE, et al. Nine residues in HLA-DQ molecules determine with susceptibility and resistance to type 1 diabetes among young children in Sweden. Sci Rep. 2021; 11(1): 8821. https://doi.org/10.1038/s41598-021-86229-8
- 34Zhao LP, Papadopoulos GK, Kwok WW, Moustakas AK, Bondinas GP, Carlsson A, et al. Next-generation HLA sequence analysis uncovers seven HLA-DQ amino acid residues and six motifs resistant to childhood type 1 diabetes. Diabetes. 2020; 69(11): 2523–2535. https://doi.org/10.2337/db20-0374
- 35Ramgopal S, Rathika C, Padma Malini R, Murali V, Arun K, Balakrishnan K. Critical amino acid variations in HLA-DQB1* molecules confers susceptibility to autoimmune thyroid disease in south India. Genes Immun. 2019; 20(1): 32–38. https://doi.org/10.1038/s41435-017-0008-6
- 36Ueki A, Isozaki Y, Tomokuni A, Ueki H, Kusaka M, Tanaka S, et al. Different distribution of HLA class II alleles in anti-topoisomerase I autoantibody responders between silicosis and systemic sclerosis patients, with a common distinct amino acid sequence in the HLA-DQB1 domain. Immunobiology. 2001; 204(4): 458–465. https://doi.org/10.1078/0171-2985-00055
- 37Klein J, Sato A. The HLA system. First of two parts. N Engl J Med. 2000; 343(10): 702–709. https://doi.org/10.1056/NEJM200009073431006
- 38Boukouaci W, Rivera-Franco MM, Volt F, Wu CL, Rafii H, Cappelli B, et al. Comparative analysis of the variability of the human leukocyte antigen peptide-binding pockets in patients with acute leukaemia. Br J Haematol. 2023; 200(2): 197–209. https://doi.org/10.1111/bjh.18517
- 39James EA, Moustakas AK, Bui J, Nouv R, Papadopoulos GK, Kwok WW. The binding of antigenic peptides to HLA-DR is influenced by interactions between pocket 6 and pocket 9. J Immunol. 2009; 183(5): 3249–3258. https://doi.org/10.4049/jimmunol.0802228
- 40Xie LJ, Cui Z, Chen FJ, Pei ZY, Hu SY, Gu QH, et al. The susceptible HLA class II alleles and their presenting epitope(s) in Goodpasture's disease. Immunology. 2017; 151(4): 395–404. https://doi.org/10.1111/imm.12736
- 41Voorter CE, Amicosante M, Berretta F, Groeneveld L, Drent M, van den Berg-Loonen EM. HLA class II amino acid epitopes as susceptibility markers of sarcoidosis. Tissue Antigens. 2007; 70(1): 18–27. https://doi.org/10.1111/j.1399-0039.2007.00842.x
- 42Menconi F, Osman R, Monti MC, Greenberg DA, Concepcion ES, Tomer Y. Shared molecular amino acid signature in the HLA-DR peptide binding pocket predisposes to both autoimmune diabetes and thyroiditis. Proc Natl Acad Sci USA. 2010; 107(39): 16899–16903. https://doi.org/10.1073/pnas.1009511107
- 43Hu X, Deutsch AJ, Lenz TL, Onengut-Gumuscu S, Han B, Chen WM, et al. Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat Genet. 2015; 47(8): 898–905. https://doi.org/10.1038/ng.3353
- 44Ishigaki K, Lagattuta KA, Luo Y, James EA, Buckner JH, Raychaudhuri S. HLA autoimmune risk alleles restrict the hypervariable region of T cell receptors. Nat Genet. 2022; 54(4): 393–402. https://doi.org/10.1038/s41588-022-01032-z
- 45Zhao LP, Skyler J, Papadopoulos GK, Pugliese A, Najera JA, Bondinas GP, et al. Association of HLA-DQ heterodimer residues -18β and β57 with progression from islet autoimmunity to diabetes in the diabetes prevention trial-type 1. Diabetes Care. 2022; 45(7): 1610–1620. https://doi.org/10.2337/dc21-1628
- 46Zhou F, Cao H, Zuo X, Zhang T, Zhang X, Liu X, et al. Deep sequencing of the MHC region in the Chinese population contributes to studies of complex disease. Nat Genet. 2016; 48: 740–746. https://doi.org/10.1038/ng.3576
- 47Bondinas GP, Moustakas AK, Papadopoulos GK. The spectrum of HLA-DQ and HLA-DR alleles, 2006: a listing correlating sequence and structure with function. Immunogenetics. 2007; 59(7): 539–553. https://doi.org/10.1007/s00251-007-0224-8
- 48Hov JR, Kosmoliaptsis V, Traherne JA, Olsson M, Boberg KM, Bergquist A, et al. Electrostatic modifications of the human leukocyte antigen-DR P9 peptide-binding pocket and susceptibility to primary sclerosing cholangitis. Hepatology. 2011; 53(6): 1967–1976. https://doi.org/10.1002/hep.24299
- 49Nowak J, Wozniak J, Mendek-Czajkowska E, Dlugokecka A, Mika-Witkowska R, Rogatko-Koros M, et al. Potential link between MHC self-peptide presentation and hematopoiesis; the analysis of HLA-DR expression in CD34-positive cells and self-peptide presentation repertoires of MHC molecules associated with paroxysmal nocturnal hemoglobinuria. Cell Biochem Biophys. 2013; 65(3): 321–333. https://doi.org/10.1007/s12013-012-9435-149
- 50Shichishima T, Okamoto M, Ikeda K, Kaneshige T, Sugiyama H, Terasawa T, et al. HLA class II haplotype and quantitation of WT1 RNA in Japanese patients with paroxysmal nocturnal hemoglobinuria. Blood. 2002; 100: 22–28. https://doi.org/10.1182/blood.v100.1.22
- 51Lombardi ML, Terrazzano G, Cosentini E, Gargiulo L, Risitano A, Camerlingo R, et al. Paroxysmal nocturnal hemoglobinuria: significant association with specific HLA-A, -B, -C, and -DR alleles in an Italian population. Hum Immunol. 2008; 69: 202–206. https://doi.org/10.1016/j.humimm.2008.02.001
- 52Giudice V, Selleri C. Aplastic anemia: pathophysiology. Semin Hematol. 2022; 59(1): 13–20. https://doi.org/10.1053/j.seminhematol.2021.12.002
- 53Yoshizato T, Dumitriu B, Hosokawa K, Makishima H, Yoshida K, Townsley D, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015; 373(1): 35–47. https://doi.org/10.1056/NEJMoa1414799
- 54Sun L, Babushok DV. Secondary myelodysplastic syndrome and leukemia in acquired aplastic anemia and paroxysmal nocturnal hemoglobinuria. Blood. 2020; 136(1): 36–49. https://doi.org/10.1182/blood.2019000940
- 55Binkowski TA, Marino SR, Joachimiak A. Predicting HLA class I non-permissive amino acid residues substitutions. PLoS One. 2012; 7(8):e41710. https://doi.org/10.1371/journal.pone.0041710
- 56Pagliuca S, Gurnari C, Awada H, Kishtagari A, Kongkiatkamon S, Terkawi L, et al. The similarity of class II HLA genotypes defines patterns of auto reactivity in idiopathic bone marrow failure disorders. Blood. 2021; 138(26): 2781–2798. https://doi.org/10.1182/blood.2021012900
- 57Angelini DF, Serafini B, Piras E, Severa M, Coccia EM, Rosicarelli B, et al. Increased CD8+ T cell response to Epstein-Barr virus lytic antigens in the active phase of multiple sclerosis. PLoS Pathog. 2013; 9(4):e1003220. https://doi.org/10.1371/journal.ppat.1003220
- 58Zdimerova H, Murer A, Engelmann C, Raykova A, Deng Y, Gujer C, et al. Attenuated immune control of Epstein-Barr virus in humanized mice is associated with the multiple sclerosis risk factor HLA-DR15. Eur J Immunol. 2021; 51(1): 64–75. https://doi.org/10.1002/eji.202048655
- 59Nakao S, Takamatsu H, Chuhjo T, Ueda M, Shiobara S, Matsuda T, et al. Identification of a specific HLA class II haplotype strongly associated with susceptibility to cyclosporine-dependent aplastic anemia. Blood. 1994; 84(12): 4257–4261. https://doi.org/10.1182/blood.V84.12.4257.bloodjournal84124257
- 60Chen L, Ge M, Huo J, Ren X, Shao Y, Li X, et al. Association between human leukocyte antigen and immunosuppressive treatment outcomes in Chinese patients with aplastic anemia. Front Immunol. 2023; 14:1056381. https://doi.org/10.3389/fimmu.2023.1056381
- 61Fu RT, Xue HM, Zhang BH, Wang J, Lin SF, Chen C. Correlation analysis of severe aplastic anemia immunosuppressive therapy and human leukocyte antigen alleles in pediatric patients. Exp Ther Med. 2015; 10(6): 2396–2402. https://doi.org/10.3892/etm.2015.2807