Deciphering molecular complexity of HCV-associated lymphoproliferation
Abstract
Recent clinical studies demonstrated the achievement of lymphoma responses in patients with Hepatitis C virus-associated indolent lymphoproliferative disorders (LPD) receiving direct-acting antivirals (DAAs) as their sole treatment. However, the molecular mechanisms underlying LPD responses to DAAs are still poorly understood. In their paper the authors provide new molecular insights on this issue, reporting intraclonal diversification and persistence of B-cell clones in most cases, despite viral eradication and beneficial clinical outcome. These provocative data suggest that the achievement of molecular response is probably not required for a ‘functional cure’ of these patients. Further comprehensive immunogenetic and mutational studies would be fundamental to dissect this biological puzzle and, ultimately, to refine improved treatment strategies in this setting.
Commentary on: Mazzaro et al. Persistence of monoclonal B-cell expansion and intraclonal diversification despite virus eradication in patients affected by hepatitis C virus-associated lymphoproliferative disorders. Br J Haematol 2023;203:237-243.
Hepatitis C virus (HCV) virus has been associated with a wide spectrum of lymphoproliferative disorders (LPD), ranging from mixed cryoglobulinemia (MC) to overt B-cell lymphomas, especially indolent subtypes, mostly represented by marginal-zone lymphomas (MZL), and diffuse large B-cell lymphomas.1
The most compelling evidence supporting the aetiological role of HCV in sustaining B-cell lymphoproliferation is represented by clinical studies reporting the regression of indolent LPD after HCV clearance induced by antiviral therapy with novel direct-acting antivirals (DAAs). In fact, differently from seminal studies using interferon (IFN), which is endowed of anti-proliferative properties,2 the successful demonstration of lymphoma regression of HCV-positive indolent LPD after DAAs,3 which act by targeting specific viral proteins, made evident that these durable responses should be ascribed to per se HCV eradication. Notably, although overall and complete response rates of seemed to be lower compared with studies evaluating IFN-based regimens, progression-free survival (PFS) rates appear superimposable2, 3 (Table 1). In particular, the recently published prospective ‘BArT study’, led by the Fondazione Italiana Linfomi, demonstrated that about half of patients with HCV-positive indolent lymphoma receiving DAAs as the sole primary treatment achieved at least a partial lymphoma regression (complete in 20%), which resulted quite durable, with a 3-year PFS of 76%.4 These results confirmed with a high level of evidence that DAAs should be recommended as first-line treatment in all patients with HCV-associated indolent LPD, at least in cases when a conventional treatment is not urgent.6
| Reference, year | Study type | Countries | Patients, N | Histotypes | Antiviral therapy | SVR, % | ORR (CR), % | 3y-PFS, % | Median f-up |
|---|---|---|---|---|---|---|---|---|---|
| Arcaini et al., 20142 | Retrospective | Italy | 100 | MZL n = 60 | IFN/pegIFN + RBV | 80 | 77 (44) | 79 | 3.6 years |
| MZL n = 40 | |||||||||
| Frigeni et al., 20203 | Retrospective | Italy, France, the US and Germany | 66 | MZL n = 53 | DAAs | 98 | 69 (21) | 74 | 1.3 years |
| Non-MZL n = 13 | |||||||||
| Merli et al., 20224 | Prospective (BArT study) | Italy | 40 | MZL n = 27 | DAAs | 100 | 45 (20) | 76 | 3.1 years |
| Non-MZL n = 13 | |||||||||
| Mazzaro et al., 20235 | Retrospective | Italy | 23 | MZL n = 11 | DAAs | 100 | 13 (4) | 73 | 3.0 years |
| Non-MZL n = 4 | |||||||||
| MBL n = 8 |
- Abbreviations: CR, complete response; DAAs, direct-acting antivirals; f-up, follow-up; HCV, hepatitis C virus; IFN, interferon; LPD, lymphoproliferative disorders; MBL, monoclonal B-cell lymphocytosis; MZL, marginal-zone lymphomas; ORR, overall response rate; pegIFN, pegylated interferon; PFS, progression-free survival; RBV, ribavirin; SVR, sustained virologic response.
Although the introduction of DAAs opened a new era in the treatment of HCV-positive indolent LPD, the molecular mechanisms underlying LPD responses achieved by using this ‘pathogenic targeted therapy’, as well as the molecular features associated with responses, are still poorly elucidated.
In their paper the authors5 provided new stimulating molecular data on this issue. Briefly, the authors reported an extensive pre- and post-DAAs characterization by IGHV and BCL2/IGH rearrangements of a series of 23 patients with HCV-associated LPD, including eight monoclonal B-cell lymphocytosis (MBL) and 15 overt indolent lymphomas (11 MZL). Interestingly, the analysis of the heavy-chain complementarity determining region 3 (HCDR3) sequences of B-cell receptor (BCR) revealed that five cases carried stereotyped HCRD3 with a strict similarity to those of known rheumatoid factor (RF)-producing LPD, while other six cases had HCDR3 resembling each other, suggesting the recognition of yet unidentified HCV-related antigen(s). Moreover, a bioinformatic analysis of subclones revealed a certain degree of intraclonal diversification, suggestive of a continuous antigen-driven B-cell stimulation due to HCV.
All patients received DAAs per clinical practice and achieved a sustained virologic response. A disappearance of serum cryoglobulins with the resolution of MC-related vasculitis symptoms was detected in most cases. However, only three patients achieved an objective response (13%), whereas a disappearance of the peripheral blood B-cell clone was detected in only two out of 19 evaluable patients (molecular response 11%), both receiving other treatments in addition to DAAs (rituximab and IFN). Interestingly, in four cases the major baseline clone substantially decreased or was replaced by novel clones after DAAs, suggesting that cessation of antigen stimulation by HCV may have been responsible for clonal variation. At the last follow-up, none of the patients with MBL evolved into overt lymphoma and only three ultimately progressed, with a 3-year PFS of 73%.
Taking into account cautions related to the small sample size, these results are very provocative and raise many questions. First, the observation of the intraclonal diversification and stereotyped HCDR3 add other unequivocal confirmations about the stepwise model of antigen-driven HCV-related lymphoproliferation (Figure 1). However, the demonstration through molecular methods of the persistence of B-cell clones in the majority of cases, despite viral eradication and cryoglobulin clearance, seems to suggest that in the context of indolent HCV-related LPD treated with DAAs, the achievement of molecular response is not strictly required for a ‘functional cure’. This is in keeping with the parallel scenario of gastric MALT lymphoma after Helicobacter pylori eradication by antibiotics, where monoclonal B cells are frequently detected by molecular studies, that is IGHV rearrangement, for long times also after the demonstration of complete histologic response, without any clear relation with increased risks of progression.7 Furthermore, clinical experiences reporting the use of DAAs as primary treatment in HCV-related indolent LPD highlighted that also the achievement of clinical complete response may not be considered critical for a long-term favourable outcome, given that despite low response rates, about 75% of patients remained progression-free at 3 years of follow-up. Speculative explanations of these findings include the cessation of antigen-mediated activating signals through BCR, and the reduction in acquired secondary genetic lesions in lymphoma cells.8
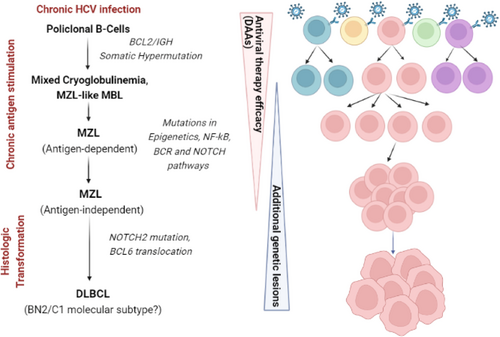
The second important issue raised by this study is represented by the investigation of molecular and immunogenetic signatures that may be associated with differential likelihood of LPD response after DAAs-induced HCV eradication. Notably, although it was not possible to draw definitive conclusions, some HCDR3s putatively shaping anti-HCV or anti-RF specificity of BCR seemed to be associated with signals of increased LPD responsiveness. At this purpose, recent studies suggested that besides HCDR3, stereotyped κ light-chain CDR3 (KCDR3) enriched in sequences homologous to anti-HCV E2 antibodies may be detected at high frequency in HCV-positive LPD (54%–70%) and, intriguingly, resulted significantly associated with higher probability to achieve a haematological response after DAAs compared with non-stereotyped KCDR3.9, 10
Finally, another fundamental piece of this multifaceted puzzle was provided by the evaluation of mutational signatures of HCV-positive LPD, which have been recently explored.10, 11 Interestingly, it was shown that patients exhibiting stereotyped KCDR3 were enriched in mutations involving pathways regulating epigenetics, NF-kB, BCR and NOTCH signalling, compared with those carrying non-stereotyped KCDR3.10 This may suggest that a subset of patients may harbour distinct mutational and immunogenetic signatures associated with pronounced sensitivity to HCV eradication.
In conclusion, future translational studies including multidimensional molecular analyses of sequential samples of patients with HCV-positive LPD treated with DAAs are eagerly awaited to definitely dissect this biological puzzle. Further molecular insights may also ultimately allow to refine improved therapeutic strategies, likely involving concurrent or sequential combination of DAAs with targeted agents, such as anti-CD20 monoclonal antibodies or BTK inhibitors.




