Fetal and neonatal alloimmune thrombocytopenia: No evidence of systemic inflammation as a modulator of disease severity. Could placental inflammation be key?
David Böhm and Sandra Wienzek-Lischka contributed equally to this study.
Summary
In fetal/neonatal alloimmune thrombocytopenia (FNAIT), maternal alloantibodies against paternal human platelet antigens (HPA) cross the placenta and lead to platelet destruction. The extent of thrombocytopenia varies among neonates, and inflammation may constitute an important trigger. A set of stable inflammatory markers was measured in serum samples from neonates with low platelet counts, of which n = 50 were diagnosed with FNAIT due to anti-HPA-1a antibodies and n = 50 were thrombocytopenic without detectable maternal HPA antibodies. Concentrations of C-reactive protein, soluble CD14, procalcitonin, and sFlt-1 did not differ between the two cohorts. There was no correlation between C-reactive protein or soluble CD14 and the platelet count, but a negative correlation between procalcitonin concentrations and the neonatal platelet count in both cohorts. sFlt-1 concentration and the platelet count were correlated in FNAIT cases exclusively. None of the inflammatory markers was statistically different between cases with and without intracranial haemorrhage. We were unable to identify systemic inflammation as a relevant factor for thrombocytopenia in FNAIT. The antiangiogenic enzyme sFlt-1, released by the placenta, did correlate with the platelet count in FNAIT cases. Our findings may give rise to the hypothesis that placental inflammation rather than systemic inflammation modulates disease severity in FNAIT.
INTRODUCTION
Fetal/neonatal alloimmune thrombocytopenia (FNAIT) is caused by maternal IgG alloantibodies which cross the placenta and bind to fetal platelets. IgG-decorated platelets are then destroyed mainly through phagocytes in the spleen and liver, resulting in thrombocytopenia. The maternal immune response in Caucasians is mainly directed against human platelet antigen (HPA) 1a.1, 2 Approximately 2000 out of 100 000 pregnancies occur in women who do not carry the HPA-1a antigen; 200 of these women will be immunized. In 60 cases, the resulting fetal or neonatal thrombocytopenia will be severe (<50 × 109/L) and of these, approximately 6 will experience intracranial haemorrhage (ICH).3 Some correlation between the antibody level and the platelet decrement has been reported,4 but cases with high antibody levels and normal platelet counts, as well as cases with low antibody levels and critically low platelet counts, are observed regularly. Apparently, the presence of anti-HPA-1a antibodies is not enough to explain the extent of thrombocytopenia. Potential modulators of FNAIT severity have been reviewed recently.5 Among these modulators, inflammation affecting the fetus has attracted growing attention. It is well known that inflammatory co-stimulation can alter the efficacy of immune responses and it is conceivable that, in FNAIT, comparable antibody levels may exert different effects on platelets if inflammatory co-stimulators are present. To our knowledge, only C-reactive protein (CRP) has been investigated in FNAIT babies so far.6 CRP is an acute-phase protein produced by hepatocytes in response to inflammatory cytokines. It is a well-established marker for neonatal sepsis. Beyond this, HPA-1a positive platelets, after binding anti-HPA-1a antibodies, expose phosporylcholine, which is a binding partner for CRP.6 The authors of this study could demonstrate in vitro that if the number of anti-HPA-1a IgG attached to the platelet surface by itself is too low to stimulate phagocytosis, elevated CRP levels stand in to allow sufficient platelet removal. Since elevated CRP levels are often observed together with other inflammatory markers, we conducted this retrospective study on blood samples from 100 newborns with a suspected diagnosis of FNAIT. In addition to CRP, soluble CD14 (sCD14), procalcitonin (PCT), and soluble Flt-1 (sFlt-1) levels were analysed and compared with the platelet count and the occurrence of ICH.
Soluble CD14 is regarded as an overall marker of monocyte/macrophage activation. In the presence of inflammatory cytokines, the soluble form is generated by cleavage from the surface and/or release from intracellular pools.7 We included this marker based on the hypothesis that in FNAIT, antibody-loaded platelets are removed from the circulation by phagocytosis, which involves monocyte/macrophage activation.
PCT is considered a more sensitive marker of bacterial inflammation than CRP.8 PCT was therefore included in our panel in order to identify potential interference of early-onset bacterial infections. However, in addition to its role as a biomarker, PCT has also been reported to induce direct damage to endothelial cells and to exacerbate endothelial dysfunction.9 This observation led us to include sFlt-1 as an additional endothelial marker because sFlt-1 has been reported to be equivalent to the signature cytokine of inflammation, IL-6,10 and also to represent a marker of endothelial damage.11 The consideration of biomarkers that are associated with inflammation and simultaneously with endothelial cell damage is also interesting in light of the fact that at least for the most severe form of FNAIT, ICH, mechanisms of direct damage to endothelial cells by antibodies against HPA-1a have been suspected.12
MATERIALS AND METHODS
Patient cohort
Suspected FNAIT cases sent for laboratory work-up were retrospectively screened for inclusion. We excluded cases with maternal systemic lupus erythematosus (SLE) and/or immune thrombocytopenia (ITP), cases with pre-eclampsia and newborns with signs of systemic inflammation, sepsis, and necrotizing enterocolitis as well as newborns with malformations. We also excluded cases with a neonatal platelet count above 150 G/L. Suspected diagnosis given by the responsible physician, week of gestation and birth weight were recorded when available.
Neonatal and maternal platelet counts
Platelet counts were determined from EDTA blood samples using a standard haematology analyser (Sysmex kx21N). Platelet counts <10 × 109G/L were controlled microscopically using a counting chamber.
Genetic and serological testing
DNA was isolated from maternal, paternal and neonatal whole blood and genotyped for HPA-1, −2, −3, −5, −9, and −15 by real-time PCR (TaqMan, Applied Biosystems). Maternal serum was used for the detection of platelet-reactive alloantibodies. Paternal platelets and platelets from donors with known HPA genotypes were isolated for an indirect monoclonal antibody immobilization of platelet antigens (MAIPA) assay.13 Maternal platelets were isolated from mothers with thrombocytopenia (platelet count <100 × 109/L) for detection of maternal autoantibodies in a direct MAIPA. MAIPA crossmatch (maternal serum against paternal platelets) was performed using monoclonal antibodies (MoAbs) Gi5, FMC25, Gi9, TAE/D2 and Gi18 specific for platelet membrane glycoproteins (GP) GP IIb/IIIa, GP Ib/IX, GP Ia/IIa, CD 109, and CD31, respectively. Maternal serum was also tested with at least three-panel cells of known genotype in an indirect MAIPA with the same set of MoAbs. Test sensitivity was controlled with the World Health Organization anti-HPA-1a reference reagent (NIBSC 05/106). Direct MAIPA was performed with MoAbs Gi5, FMC25 and Gi9 against GPs IIb/IIIa, Ib/IX, and Ia/IIa to exclude maternal autoantibodies as a cause of low neonatal platelet counts.
Luminex assay
Neonatal serum samples were investigated for the presence of inflammatory markers. Low-volume Luminex testing was performed as recommended by the manufacturer (R&D systems) using a Luminex 200 detection system (Luminex Corporation). Briefly, 50 μL of pooled colour-coded beads, pre-coated with analyte-specific capture antibodies were incubated with 50 μL neonatal serum (using 1:2 dilution for sFlt-1 and procalcitonin testing; or 1:200 dilution for CRP and sCD14- testing, as recommended by the manufacturer) for 120 min, and then washed four times with wash buffer. Next, 50 μL of biotinylated detection antibodies specific to the analytes were added, and incubated for 60 min to form an antibody–antigen sandwich. Afterwards, 50 μL phycoerythrin (PE)-conjugated streptavidin was added, binding the biotinylated detection antibodies. After 30 min of incubation, samples were washed three times, and the probes were measured. Standard preparations for all inflammation markers were provided by the manufacturer.
Statistical analysis
Statistics were performed using GraphPad Prism 7 Software. Spearman rank test was used for the calculation of a p-value, considering p-values <0.05 significant.
Ethics statement
The study was approved by the Ethics Committee of the Medical Faculty, Justus-Liebig-University Giessen, Germany (file number 82/09, 07.04.2014).
RESULTS
Comparison of the two cohorts
We included 50 families with confirmed FNAIT of HPA-1a positive newborns due to maternal anti-HPA-1a antibodies; and 50 families with thrombocytopenic newborns in whom FNAIT was excluded in the platelet immunology laboratory. Suspected diagnoses in thrombocytopenic newborns without FNAIT were recorded for 27 cases, with asphyxia (n = 7), trisomy 21 (n = 6) and intrauterine growth retardation (n = 4) being the most common. Gender distribution was comparable between the FNAIT and the non-FNAIT group, with female newborns being underrepresented in both cohorts (Table 1). The number of newborns with ICH and other bleeds was significantly higher in the FNAIT group. The platelet count was significantly lower in FNAIT cases compared to non-FNAIT cases. Cohorts did not differ for week of gestational age, birth weight, and number of small for gestational age (SGA) babies. Serum concentrations of CRP, sCD14, PCT, and sFlt-1 did not differ between thrombocytopenic newborns with and without FNAIT.
| Characteristics | FNAIT | Non-FNAIT | p |
|---|---|---|---|
| Total number | 50 | 50 | |
| Gender, male/female | 38/12 | 34/16 | 0.373 |
| ICH (n) | 11 | 1 | 0.002 |
| Other bleeds (n) | 22 | 3 | <0.001 |
| Week of gestation (median and range)a | 39 (36–41) | 39 (36–42) | 0.058 |
| SGA (n/total n)a | 9/27 (33%) | 4/25 (16%) | 0.149 |
| Birth weight (g)a | 3159 (2810–3650) | 3213 (2900–3595) | 0.718 |
| Platelet count (G/L) | 20 (11–42) | 45 (28–72) | <0.001 |
| CRP (mg/L) | 4.78 (1.48–8.25) | 4.44 (1.86–5.76) | 0.964 |
| sCD14 (μg/mL) | 6.44 (5.36–8.55) | 8.18 (5.98–9.66) | 0.427 |
| Procalcitonin (μg/L) | 0.36 (0.26–0.79) | 0.32 (0.18–0.48) | 0.133 |
| sFlt-1 (pg/mL) | 454.5 (324.5–744.75) | 401.8 (277–474.4) | 0.243 |
- Note: Continuous values are given as mean, 25th, and 75th percentile, if not indicated otherwise. Comparisons between unpaired groups were performed by Mann–Whitney rank sum test and χ2-test.
- Abbreviations: CRP, C-reactive protein; FNAIT, fetal/neonatal alloimmune thrombocytopenia; ICH, intracranial haemorrhage.
- a Values for week of gestation and birth weight were not available for 23/50 children in the FNAIT group and for 25/50 children in the non-FNAIT group. Small for gestational age (SGA), defined as a birth weight of less than 10th percentile for gestational age, was calculated for newborns with complete data.
Correlation between inflammatory markersand the neonatal platelet count
In order to assess relationships between inflammatory markers and the neonatal platelet count, Spearman correlation coefficients were calculated (Figure 1). There was no correlation between CRP concentrations and the platelet count in thrombocytopenic newborns with (rs = 0.149, p = 0.306) or without (rs = 0.004, p = 0.975) FNAIT and no correlation between sCD14 and the platelet count in thrombocytopenic newborns with (rs = 0.117, p = 0.415) or without (rs = 0.082, p = 0.572) FNAIT. A moderate negative correlation was observed between PCT concentrations and the neonatal platelet count in both cohorts: rs = −0.39, p = 0.006 for thrombocytopenic newborns with FNAIT and rs = −0.378, p = 0.007 for thrombocytopenic newborns without FNAIT. In addition, we observed a discrepancy between the two cohorts when assessing the correlation between sFlt-1 concentration and the platelet count. Whereas a moderate negative correlation was observed in FNAIT cases (rs = −0.388, p = 0.005), no correlation could be demonstrated in the non-FNAIT cohort (rs = −0.065, p = 0.652).
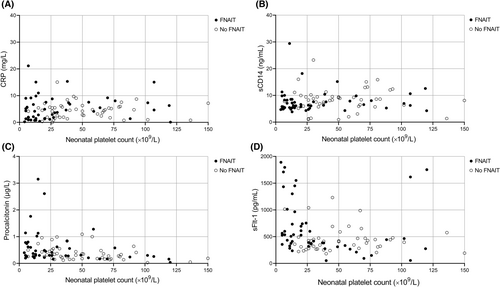
Correlation between inflammatory markers and ICH
Within the FNAIT cohort (n = 50), 11 newborns were diagnosed with ICH, and 39 did not have intracranial bleeding. The platelet count was significantly different between these two subgroups: 15 G/L (7–23) for FNAIT babies with ICH versus 38 G/L (13–57) for FNAIT babies without ICH, p = 0.034. The birth weight was also significantly different between the two subgroups (2863 g (2660–3000) vs. 3293 g (2958–3663), p = 0.032), whereas they did not differ for gestational weeks (p = 0.059) and number of SGA babies (p = 0.083). None of the inflammatory markers was statistically different between the two subgroups (CRP, p = 0.293; sCD14, p = 0.936; procalcitonin, p = 0.697; and sFlt-1, p = 0.968).
DISCUSSION
We were unable to obtain evidence for the impact of fetal/neonatal inflammation on the neonatal platelet count when comparing thrombocytopenic newborns with and without FNAIT. CRP, which presumably can support the phagocytosis of antibody-loaded platelets,6 was not different between cases and controls. This observation is in contrast to findings by others, who compared newborns with FNAIT with a healthy control group (21 individuals per cohort) found a difference.6 One explanation for our observation could be that our control cohort, which was thrombocytopenic, included significant numbers of newborns with undetected infections, whereas a healthy non-thrombocytopenic control group was used in the other study. We do not think that this is the case since we excluded newborns with evidence of infection from the study. There is no generally accepted normal range for CRP in newborns, and both our cohorts, with no statistical difference between them, were either in-range or slightly above expected concentrations, depending on the reference interval.14, 15 However, almost all newborns (92/100) had CRP levels below the most often applied cut-off value of 10 mg/dL.16 In addition, CRP levels and platelet counts did not correlate in our cohorts, whereas this is to be expected in newborns with infections.17 CRP levels are also known to be modified by several additional perinatal factors and infant characteristics, for which both our study and the study by Kapur were not controlled.18 We also found no correlation between CRP levels and the occurrence of ICH, in accordance with Kapur's study.
Soluble CD14 is regarded as an overall marker of monocyte/macrophage activation and is also a useful marker for sepsis of the newborn.19 We found clearly higher sCD14 concentrations in comparison to published data,20, 21 but again no differences between the groups. We did expect a possible increase in FNAIT when compared to non-FNAIT thrombocytopenia since antibody-loaded platelets are usually removed from the circulation by phagocytosis, which involves monocyte/macrophage activation, but no evidence of that could be retrieved from our data. There was also no correlation between sCD14 and platelet counts in the two cohorts.
PCT is secreted ubiquitously in response to endotoxin or inflammatory mediators and correlates with the severity of bacterial infections.22 It is therefore used as a diagnostic marker of neonatal sepsis.23 PCT concentrations are modified by birth weight, age of gestation, and the time point of the rupture of the membranes.8 In our study, PCT concentration, in contrast to CRP, showed a general correlation with the platelet count for all neonates. However, there was no difference observed between the FNAIT and non-FNAIT groups. A negative correlation between PCT and the platelet count in newborns (rs = −0.317), somewhat comparable with our results, has been reported previously.24 There are two potential explanations for this correlation: the platelet mass itself may affect the (measurable) PCT concentration. Or, elevated levels of PCT may indicate a modulatory role of inflammation, affecting neonatal platelet counts independent of the mechanism (FNAIT or non-FNAIT). In this regard, it should be mentioned that PCT has also been identified as a promising parameter for the prediction of chorioamnionitis.25 However, whereas PCT levels were correlated with the platelet count in each cohort, we did not observe a statistically significant difference in PCT concentrations between the cohorts.
Finally, sFlt-1 is a marker of endothelial dysfunction.10 We observed a moderate negative correlation between sFlt-1 concentration and the platelet count in FNAIT cases (rs = −0.388, p = 0.021), but not in the non-FNAIT cohort (rs = −0.065, p = 1). Whereas sFlt-1 is considered to represent a vascular endothelial marker in adult sepsis, there is currently not enough data available for neonates.26 We do not think that sepsis (or bacterial inflammation) explains the observed correlation between sFlt-1 and platelet counts in the FNAIT cohort, first, because medical records did not indicate inflammation in any of the cases included in this study, and second, because there was no correlation observed between CRP or sCD14 and the platelet count. This largely excludes bacterial infections as triggers for sFlt-1.
Another, non-endothelial source for sFlt-1 could be the placenta. It is well established that during pregnancy, endometrial VEGF directly induces the production and release of sFlt-1 from placental tissues.27 In premature neonates of pre-eclamptic mothers, Tsao et al. have shown that the sFlt-1 concentration was increased in cord blood samples and that sFlt-1 was significantly and independently related to the neonatal platelet count.28 Pre-eclampsia does not explain our observation, since pre-eclamptic mothers were excluded from the study. But, more recent data obtained in a rodent model are indicating that placental inflammation also triggers Flt-1 and, in rats, increases offspring death rate and low birth weight.29 Whether antibody-associated, “sterile” placentitis described in FNAIT cases30, 31 also leads to increased release of sFlt-1 has not been investigated. Unfortunately, placenta material from individuals included in our study was not available. The design of our study does not allow for the identification of the exact cellular source of sFlt-1 in neonatal blood. What is well known is that sFlt-1 prevents the interaction between VEGF and its receptor on megacaryocytes and significantly inhibits megacaryocyte polyploidization.32 Accordingly, sFlt-1, independently of its cellular source, may further aggravate thrombocytopenia in FNAIT. It is intriguing to speculate that other clinical observations that “overlap” between newborns from pre-eclamptic mothers and newborns with FNAIT such as low birth weight, may also be (at least partially) attributed to sFlt-1.33 Only recently, de Vos et al., investigating placental complement activation in FNAIT, described a higher degree of C4d deposition in newly diagnosed FNAIT cases, as compared to the IVIg-treated FNAIT cases or negative controls. They concluded that a higher degree of classical pathway-induced complement activation is present in placentas from pregnancies with untreated FNAIT, which may affect placental function and fetal growth.34 Further research shall aim to combine indicators of placental inflammation and dysfunction in FNAIT in order to increase our understanding of how the fetal-placental unit modifies disease severity in this disorder.
Of note, we did not observe any correlation between the inflammatory markers and the occurrence of ICH. It may well be that (placental) inflammation affects the fetal/neonatal platelet count but does not influence processes required to precipitate ICH such as antibody-mediated anti-endothelial activity.5
Our study has several limitations. The FNAIT group and the control group were matched for plasma storage time, but not for obstetric or neonatal aspects. It was also designed to investigate the effect of systematic inflammation rather than the effect of placental processes. Although gestational age and birth weight, both of which may have influenced the parameters obtained, were only available for about half the cases and controls, we believe that the study is still suitable for drawing appropriate, preliminary conclusions. In addition, the study would of course benefit from data on placental histology, but no such material was available for evaluation.
In conclusion, we were unable to find evidence that systemic inflammation modulates disease severity in FNAIT specifically. This does not necessarily mean that systemic inflammation is irrelevant to the extent of thrombocytopenia in FNAIT, since we observed a correlation between PCT levels and platelet counts in thrombocytopenic newborns, but this was observed in the FNAIT and the non-FNAIT cohort.
However, the observed correlation between placenta-associated sFlt-1 levels and neonatal platelet counts exclusively in FNAIT newborns indicates that placental inflammation could play a key role in FNAIT disease severity.
AUTHOR CONTRIBUTIONS
David Böhm, Sandra Wienzek-Lischka and Nina Cooper collected and analysed data and helped writing the manuscript. Heike Berghöfer, Katja Müller and Behnaz Bayat performed experiments. Gregor Bein and Behnaz Bayat analysed data and performed statistics. Ulrich J. Sachs designed the study, analysed data, and wrote the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the technical expertise of Astrid Giptner-Rieger, Marica Müller, Claudia Zwingel, Sabine Paulus and Christina Schön in the platelet immunology laboratory and the valuable support of Anette Kirsch-Altena with the preparation of this manuscript. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The study was funded in part by LOEWE exploration, the research excellence initiative for the future of Hessen, through the Hessian State Ministry of Higher Education, Research and the Arts (to B.B. and U.J.S.).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to disclose.
PATIENT CONSENT STATEMENT
The design of the study did not create an obligation to obtain informed consent.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.




