Persistence of monoclonal B-cell expansion and intraclonal diversification despite virus eradication in patients affected by hepatitis C virus-associated lymphoproliferative disorders
Summary
We investigated 23 hepatitis C virus (HCV)-infected patients with overt lymphoproliferative diseases (15 cases) or monoclonal B lymphocytosis (8 cases) treated with direct agent antiviral (DAAs) per clinical practice. DAA therapy yielded undetectable HCV-RNA, the complete response of cryoglobulinemia vasculitis and related signs, whilst the presence of B-cell clones (evaluated by flow cytometry, IGHV, and BCL2-IGH rearrangements), detected in 19/23 cases at baseline, was maintained (17/19). Similarly, IGHV intraclonal diversification, supporting an antigen-driven selection mechanism, was identified in B-cell clones at baseline and end of follow-up. DAA therapy alone, despite HCV eradication and good immunological responses, was less effective on the pathological B-cell clones.
INTRODUCTION
Hepatitis C virus (HCV) may cause several extra-hepatic manifestations, including autoimmune diseases, mixed cryoglobulinemia (MC), and lymphoproliferative disorders (LPD).1
In the case of LPD, the pathogenetic model is based on the continuous exposure of B-cell receptors (BCRs) to viral antigens due to HCV chronic infection determining a chronic antigen stimulation with subsequent antigen-dependent clonal expansion; specific genetic lesions may play a role by favouring an HCV-independent B-cell survival and proliferation.2
The histological subtypes more closely associated with HCV are marginal zone lymphoma (MZL), lymphoplasmacytic lymphoma (LPL), and, less frequently, diffuse large B-cell lymphoma (DLBCL).3 In addition, patients not developing overt LPD, may have clear documentation, in peripheral blood (PB) and/or bone marrow, of clonal B-cell populations in the absence of other signs or symptoms referred to LPD, thus fitting the diagnosis of monoclonal B lymphocytosis (MBL).4
There is evidence that therapies combining interferon (IFN) plus ribavirin (RBV) in HCV patients are associated with virological and haematological responses,4 through a mechanism mostly relying upon a direct effect of IFN on the proliferating clone rather than an indirect effect on the virus.5
In recent years, IFN-free direct antiviral agents (DAAs) have been introduced to treat HCV infection. Despite a sustained virologic response (SVR) in about 100% of cases,1 only a fraction of patients affected by HCV-related LPD obtain complete or partial haematological responses, with a minority of cases requiring other conventional treatments.6, 7 These data have been recently pinpointed in the sole available prospective study evaluating DAAs in HCV-related LPD.8
PATIENTS AND METHODS
Twenty-three HCV-infected patients with LPD/MBL were included in the study from May 2015 to May 2023. Six patients, previously reported,9-11 were included with updated follow-up. Criteria for patient selection were: LPD (including MBL) at first diagnosis or relapse, indolent course, and HCV infection (HCV-RNA positivity). HBV and HIV patients were excluded. All cases met the eligibility criteria for treatment with DAAs.12 Clinical and biological data were recorded at baseline, every 4 weeks until the end of treatment (12th–24th week), and every 3/6 months after the end of treatment (Table 1; Table S1; Figure S1). Clinical responses have been evaluated by using response evaluation criteria in lymphoma (RECIL).13
| Pts | Age/sex | HCV genotypes | MC type II/III | Pre-therapy | DAAs therapy (weeks) | Post-therapy | Follow-up post-DAA (months) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cryocrit (%) | RF (U/mL)a | C4 (mg/mL) | Cryoglobulinemic vasculitis manifestations | Liver statusb | ALT (U/L) | LPD | PB κ/λ restrictionc | BCL2/IGHd | IGHV clone | Virologic response | Cryocrit % | RF (U/mL) | C4 (mg/mL) | Cryoglobulinemic vasculitis response | ALT (U/L) | LPD responsee | BCL2/IGH | IGHV clone | Clonal response | ||||||
| 1 | 56/M | 2 | II | 4.0 | 114 | 16 | Purpura, asthenia, arthralgia, neuropathy | CH | 26 | MBL-CLL | κ | neg | 1-69 | SOF + RBV 12w | SVR | Absent | 10 | 21 | PR (neuropathy) | 18 | - | neg | Poly | Yes | 49 |
| 2 | 76/M | 2 | III | 1.5 | 12 | 22 | Purpura, asthenia, arthralgia | C | 71 | LPL | λ | neg | 4-59 | SOF + VEL 12w | SVR | Absent | 23 | 12 | CR | 23 | SD | neg | 4–59 | No | 9 |
| 3 | 76/F | 1b | II | 1.0 | 66 | 5 | Purpura, asthenia, arthralgia | CH | 21 | NMZL | κ | mcr | 3-53 | SOF + LED 12w | SVR | Absent | 12 | 5 | CR | 15 | SD | neg | 3–53 | No | 41 |
| 4 | 61/M | 1b | nd | Absent | nd | nd | Absent | CH | 17 | NMZL | κ | MBR | 1-2 | OMB + PAR + RTN + DAS 12w | SVR | Absent | nd | nd | na | 18 | SD | MBR | 1-2 | No | 34 |
| 5 | 72/F | 2 | nd | Absent | nd | nd | Absent | CH | 32 | SMZL | λ | MBR | 1-2 | SOF + RBV 12w | SVR | Absent | nd | nd | na | 22 | PD | MBR | 1-2 | No | 40 |
| 6 | 59/M | 3 | III | 2.0 | 56 | 8 | Purpura, asthenia, arthralgia, neuropathy | C | 81 | MBL-CLL | κ | MBR | 3-7 | SOF + DAC + RBV 24w | SVR | Absent | 10 | 19 | CR | 22 | - | MBR | 3-7 | No | 30 |
| 7 | 74/M | 1b | II | 3.5 | 25 | 14 | Purpura, asthenia, arthralgia, neuropathy | CH | 27 | MBL-MZL | κ | MBR | 3-23 | SOF + RBV 12w | SVR | Absent | 10 | 16 | CR | 25 | - | MBR | 3-23 | No | 36 |
| 8 | 78/F | 1b | II | 3.0 | 286 | 2 | Purpura, asthenia, arthralgia, neuropathy | C | 189 | MBL-MZL | κ | neg | 1-69 | SOF + RBV 24w | SVR | Absent | 92 | 2 | CR | 20 | - | neg | 1-69 | No | 41 |
| 9 | 70/F | 2 | II | 1.5 | 49 | 9 | Arthralgia | C | 27 | MBL | κ | neg | 1-69 | SOF + RBV 24w | SVR | Absent | 54 | nd | CR | 21 | - | neg | 1-69 | No | 62 |
| 10 | 69/F | 1b | III | 3.0 | 32 | 10 | Purpura | CH | 64 | SMZL | κ | nd | nd | SOF + RBV 12w | SVR | Absent | 17 | 10 | CR | 31 | SD | nd | nd | na | 14 |
| 11 | 70/M | 2 | II | 2.0 | 419 | 5 | Purpura, astenia, arthralgia | C | 105 | MBL | κ | mcr | 1-2 | SOF + RBV 24w | SVR | Absent | 100 | 16 | CR | 14 | - | mcr | 1-2 | No | 46 |
| 12 | 59/F | 1b | nd | 3.0 | >25 | 2 | Purpura, neuropathy | CH | nd | MBL-MZL | κ | neg | 1-69 | SOF + LED 12w | SVR | Absent | ≤25 | 9 | CR | 22 | - | neg | 1-69 | No | 45 |
| 13 | 52/M | 1b | nd | 6.0 | >25 | 7 | Purpura, neuropathy | CH | nd | EMZL | κ | neg | 1-2 | OMB + PAR + RTN 12w | SVR | Absent | nd | nd | CR | 14 | SD | neg | 1-2 | No | 21 |
| 14 | 78/F | 2 | nd | 3.0 | ≤25 | 5 | Purpura, neuropathy | CH | 30 | MBL-MZL | κ | neg | 1-69 | SOF + DAC 12w | SVR | Absent | nd | 13 | CR | 13 | - | neg | 1-69 | No | 29 |
| 15 | 48/F | 3 | nd | 34.0 | >25 | 13 | Purpura, neuropahty | C | 34 | NMZL | κ | MBR | 2-5 | SOF + RBV 12w | SVR | 4.0 | nd | 93 | CR | 11 | SD | neg | 2-5 | No | 69 |
| 16 | 63/F | 1b | nd | 5.0 | >25 | 4 | Neuropathy | CH | 71 | SMZL | nd | MBR | 3-30 | SOF + LED 12w | SVR | Absent | nd | 21 | PR (neuropathy) | 9 | PR | neg | 3-30 | No | 48 |
| 17 | 74/F | 1a | nd | 1.0 | nd | 20 | Absent | CH | 22 | CLL | λ | neg | 3-48 | SOF + LED 12w | SVR | Absent | nd | nd | CR | 23 | PD | neg | 3-48 | No | 33 |
| 18 | 83/M | 1b | nd | 2.0 | 2760 | 16 | Purpura, asthenia, arthralgia, neuropathy, itch | CH | 55 | LPL | nd | neg | Poly | SOF + LED 12w | SVR | 1.0 | 1480 | 25 | PR (neuropathy) | 24 | SD | neg | Poly | na | 30 |
| 19 | 80/F | 1b | nd | Absent | ≤25 | 24 | Asthenia, arthralgia, xerostomia, neuropathy, mialgia | C | 123 | NMZL | nd | MBR | 1-69 | GRAZ+ELB 12w | SVR | Absent | ≤25 | 33 | CR | 31 | SD | MBR | Poly | No | 32 |
| 20 | 69/M | 4 | nd | 0.5 | nd | nd | Raynaud, itch | CH | 118 | SMZL | nd | MBR | Poly | SOF + RBV 24wf | SVR | Absent | ≤25 | 12 | CR | 25 | SDg | neg | Poly | Yes | 50 |
| 21 | 70/F | 2 | nd | Absent | ≤25 | 16 | Neuropathy, night sweats | CH | 24 | NMZL | nd | neg | 3-23 | SOF + DAC 12w | SVR | Absent | ≤25 | 17 | PR (neuropathy) | 20 | SD | neg | 3-23 | No | 32 |
| 22 | 66/F | 2 | nd | Absent | ≤25 | 22 | Asthenia, neuropathy, itch | C | 28 | NMZL | nd | neg | Poly | SOF + RBV 12w | SVR | Absent | ≤25 | 20 | CR | 22 | SD | neg | Poly | na | 3 |
| 23 | 59/M | 3 | nd | Absent | nd | 36 | Asthenia, neuropathy, mialgia | CH | 83 | FL | nd | neg | Poly | SOF + DAC 12w | SVR | 0.5 | ≤25 | 18 | CR | 32 | PD | neg | Poly | na | 50 |
- Abbreviations: ALT, alanine transaminase; C, cyrrosis; C4, complement component four; CH, chronic hepatitis; CLL, chronic leukaemia lymphatic; CR, complete response; DAA, direct antiviral agents; DAC, daclatasvir; DAS, dasabuvir; ELB, erbasvir; EMZL, extranodal marginal zone lymphoma; F, female; FL, follicular lymphoma; GRAZ, grazoprevir; LED, ledipasvir; LPD, lymphoproliferative disorders; LPL, lymphoplasmacytic lymphoma; M, male; MBL, monoclonal B lymphocytosis; MBR, major breakpoint region; MC, mixed cryoglobulinemia; mcr, minor cluster region; na, not assessed; nd, not done; neg, negative; NMZL, nodal marginal zone lymphoma; OMB, ombitasvir; PAR, paritaprevir; PB, peripheral blood; PD, progressive disease; Poly, polyclonal IGHV detection; pos, positive; PR, partial response; RBV, ribavirin; RF, rheumatoid factor; RTN, ritonavir; SD, stable disease; SMZL, splenic marginal zone lymphoma; SOF, sofosbuvir; SVR, sustained virologic response; VEL, velpatasvir.
- a For some patients, rheumatoid factor (RF) evaluation was available only as positive (pos, i.e. >25 U/mL) or negative (neg, i.e. ≤25 U/mL).
- b CH, chronic hepatitis, as determined by fibroscan (F1 through F3); C, Cyrrosis, as determined by fibroscan (F4).
- c Peripheral blood (PB) K/L restriction, as determined by flow cytometry and/or by sequence analysis.
- d MBR, t(14;18)-major breakpoint region; mcr, t(14;18)-minor cluster region.
- e Evaluated only in overt NHLbt the RECIL criteria.
- f This patient was treated with alpha-interferon along with SOF and RBV.
- g SMZL with persistent splenomegaly.
Methods for laboratory testing, immunophenotyping, immunoglobulin sequencing and molecular evaluation of BCL2 rearrangements are detailed in Supporting Information.
RESULTS
Pre-treatment characterization
Patients' characteristics, HCV genotyping, and basal HCV-RNA levels are reported in Table S1. A MC diagnosis was done in 17/23 cases, 12 with high rheumatoid factor (RF) or low C4 levels; alanine transaminase (ALT) was elevated in 10/21 cases, and clinical manifestations of vasculitis were observed in 20/23 cases; regarding liver condition at pre-treatment, 8 cases had stage 4 fibrosis, 1 with oesophageal varices (Table 1; Table S1). As reported in Table 1 and Table S1, excluding 8 MBL (2 CLL-like, 4 MZL-like, and 2 not specified), the vast majority (11/15) was MZL.
Molecular and/or flow cytometry analyses revealed the presence of clonality in 19/23 cases (83%), as documented by IGHV gene rearrangement (18/19, 10/18 IGHV1, IGHV1-69 in 6/10; 6/18 IGHV3), κ/λ restriction (12κ/3λ) and BCL2 rearrangement (8 MBR and 2 mcr) (Table 1).
A similarity between HCDR3 sequences, as detected in BCR, to HCDR3 of known RFs was detected (cases #1, #6, #8, #9 and #19).10, 11 Moreover, cases #4, #5, #7, #11, #13 and #14 had HCDR3 resembling each other suggesting the recognition of yet unidentified (HCV-related) antigen(s) (Figure S2; Table S2).
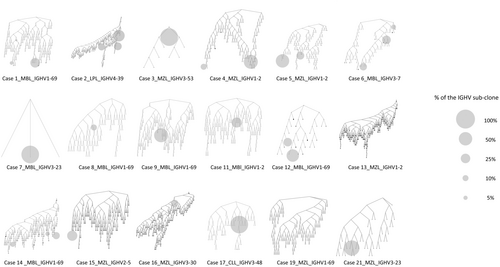
Analysing the sub-clonal composition inside each pathological clone, we observed a certain degree of intraclonal diversification (Figure 1), the phenomenon in keeping with a continuous antigen-driven B-cell stimulation due to HCV occurring both at the MBL stage and upon overt lymphoma transformation.2
DAAs treatment
All patients were treated with DAA-based therapies12 (Table 1). Thirteen cases were naïve for antiviral therapy, while 10 patients (43.5%) were non-responder/relapsed to a previous Peg-IFN/ribavirin therapy.
Patient #23, affected by FL, was treated with chemotherapy (R-CHOP) followed by anti-CD20 maintenance and consolidation with autologous PB stem cell transplant before DAA therapy. Patients #1 and #16 were treated with anti-CD20 for vasculitis-related neuropathies.
Virological and liver-related response
Upon DAAs therapy, all patients had undetectable HCV-RNA after 1 month of therapy that remained undetectable at the end of the follow-up in all cases (median follow-up 36 months; range 3–69 months) (Table 1; Figure S3A).
DAAs induced normalization of ALT serum levels, disappearance or decrease of symptoms associated to cryoglobulinemic vasculitis, and a substantial trend toward normalization of the associated biochemical hallmarks (Table 1; Figure S1). Patients #1, #16, #18 and #21 maintained vasculitis-related neuropathy.
Monoclonal B-cell population response
After DAA therapy, MBL clones were detected in 7/8 patients, disappearance being observed in a single patient treated with anti-CD20 (#1, Table 1), without any progression toward overt LPD during follow-up.
Among overt LPDs, stable disease (SD) was documented in 11 cases (2 LPL and 9 MZL), including patient #20 in which the PB clone (BCL2 rearrangement) disappeared, clinical partial response (PR) being achieved by a single MZL patient (#16) (Table 1). After a median follow-up of 36 months, the 3-year progression free survival was 72.7%, with three progressed patients, which initiated conventional treatments (Figure S4).
To study the effect of DAAs therapy on monoclonal B-cell populations, flow cytometry and molecular detection of IGHV rearrangements and BCL2 translocations were combined (Table 1; Figure S1). At the latest time-point from DAAs therapy, the disappearance of the PB B-cell clone was documented only in 2/19 cases (11%; case #1 and case #20), both receiving, in addition to DAAs, other treatments (Table 1).
Of note, in 12/16 cases (75%), the major specific B-cell sub-clone present at baseline was also the major B-cell sub-clone detected post-therapy (Figures S3B and S5). Only in four cases (#7, #12, #13 and #14; Figure S5), 3/4 from the IGHV1 family, we observed either a substantial decrease of the major clone(s) (#7 and #12) or the appearance of novel clone(s) at post-therapy time points (#13, #14); in these cases, clearance of antigen stimulation by HCV after DAA therapy may have been responsible for clonal variation.
DISCUSSION
Since 2015 when IFN was replaced by DAAs therapy, few studies demonstrated a certain degree of remission in indolent low-grade Non-Hodgkin's lymphoma (NHL) after HCV eradication.8, 9, 14-16
Our results demonstrated that the viral clearance, achieved in 100% of cases with good tolerability, induced haematological responses in 3/23 patients, that is, 1 MBL and 2 MZL. These data confirmed the capacity of DAA to induce a non-negligible rate of lymphoma responses, as previously reported,8 although in our cohort two of these patients received anti-CD20 therapies due to the persistence of neuropathy. At variance to previous studies,9, 16 herein we investigated the effects of DAA therapies at the molecular level by documenting the persistence of the B-cell clones bearing identical IGHV-IGHD-IGHJ rearrangements and/or the same t(14;18)-MBR/mcr translocation.
In the past, when HCV-related NHL was treated with IFN-alpha-based therapies, higher haematological responses were usually observed.5 Here, the haematologic response at the end of DAAs therapy was lower than in the past, allegedly for the lack of the direct effect of IFN-alpha on proliferating clones. Curiously, the only patient affected by overt NHL reaching a complete molecular response in the present series was an MZL (#16) treated with a DAA protocol which included IFN-alpha.
Previous studies, by investigating in HCV patients the B-cell clonality through light-chain κ/λ surface expression before and after DAAs treatment, found that circulating B-cell clones persisted in lymphoma patients for up to 2 years after HCV infection eradication,4 a κ/λ ratio normalization being observed only in patients without overt lymphoma. These results suggest that in the setting of indolent HCV-related LPD, the achievement of CR is not a critical factor for a long-term good outcome.8, 9
The molecular characterization of the BCR in HCV-associated lymphoproliferative disorders may indicate the role of the antigen in driving clonal expansion. Stereotyped BCRs have been described in HCV patients, frequently endowed with both RF activity and specificity for the HCV-E2 protein.17 In keeping with this observation, we provided evidence of HCDR3 similarities with known RF-HCDR3,11, 18 as well as of novel similarities among LPD-derived HCDR3, suggesting the recognition of yet unidentified (HCV-related) antigen(s).
Evidence has also been provided that BCR from HCV-related LPD, even at the MBL stage, may exhibit marks of ongoing mutations of IGHV genes, due to the retention, after the neoplastic transformation, of the physiological machinery for somatic hypermutation, possibly due to chronic stimulation by HCV-related antigens.2 Future studies aimed at analysing the Ig-light-chain composition and the mutational pattern of lymphomagenesis-related genes could provide novel correlations with different responses to DAAs, and enlighten the contribution of HCV to the lymphoma maintenance.10, 11
In conclusion, our results indicate that patients with HCV-associated LPD receiving DAAs therapy showed excellent SVR, low toxicity, and good cryoglobulinemic vasculitis response but unsatisfactory haematological response with persistence of molecular B-cell clonality. These data support the recommendation that patients with HCV-associated LPD should be treated with DAAs as a first line, with the objective of eradicating the virus and HCV-related non-haematological symptoms, associating, when necessary, LPD-specific therapies to manage the B-cell tumour. Trials combining the use of DAAs and LPD-specific therapies could be of help in this setting.
AUTHOR CONTRIBUTIONS
Cesare Mazzaro designed the study, interpreted data and wrote the manuscript. Filippo Vit, Erika Tissino, Federico Pozzo, Robel Papotti, Tamara Bittolo, Milvia Casato, Laura Gragnani, Antonella Zucchetto, Massimo Degan and Riccardo Bomben performed and interpreted molecular studies, and contributed to data interpretation. Filippo Vit, and Riccardo Bomben generated the bioinformatics pipeline of analysis, and performed statistical analyses. Cesare Mazzaro, Marcella Visentini, Laura Gragnani, Milvia Casato and Anna Linda Zignego collected clinical data and contributed to data interpretation. Riccardo Bomben and Valter Gattei designed the study, interpreted data and wrote the manuscript.
FUNDING INFORMATION
The present study is supported in part by Progetto Ricerca Finalizzata RF-2018-12365790, Italian Ministry of Health, Rome, Italy; Associazione Italiana Ricerca Cancro (AIRC), Investigator Grant IG-21687; FIL project GR2020 bando Giovani Ricercatori; Fondazione Giulia Maramotti e Fondazione GRADE Onlus; Associazione Italiana contro le Leucemie, linfomi e mielomi (AIL), Venezia Section, Pramaggiore/Veneto Orientale Group, Italy; Linfo-check – Bando ricerca – contributo art. 15, comma 2, lett b, LR 17/2014; “5x1000 Intramural Program”, Centro di Riferimento Oncologico, Aviano, Italy.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests.
ETHICS STATEMENT
This is a retrospective observational study approved by the Ethics Committee of Friuli-Venezia-Giulia Region (approval no. 2018-OS-147-CRO), Azienda Universitaria Policlinico Umberto I in Rome (Prot. 678/18) and University of Florence (Prot. BIO.16.014); informed consensus was obtained from all patients in accordance with the declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author.




