The clinicopathological analysis of organising pneumonia in myelodysplastic syndrome: high frequency in der(1;7)(q10; p10)
Myelodysplastic syndromes (MDS) often develop pulmonary complications such as organising pneumonia (OP) during the clinical course.1 OP is defined histopathologically by intra-alveolar buds of granulation tissue. OP can be either idiopathic or secondary which is commonly associated with autoimmune disorders infections drugs and malignancy. Although corticosteroids are effective, relapses are common after treatment cessation.2
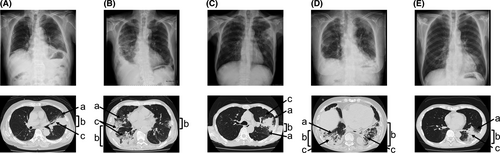
MDS accompanied by OP has been reported as case reports to date. However, little is known about its frequency, pathogenesis, and impacts on clinical features of MDS. To address these questions, we retrospectively investigated 159 patients with MDS in our institute. We included in the samples our patients with MDS whom we diagnosed from 1 January 2010 to 30 September 2017. The detailed methods, such as study design, patients, and statistical analysis are described in Data S1. Five patients (3·1%) developed pathologically diagnosed OP. Their chest imaging examinations are shown in Fig 1. Subpleural distributed consolidation with air bronchogram and ground-glass opacities were observed in all cases. Bilateral or multiple recurrent or migratory lung lesions were observed over time. The specimens of all patients obtained by transbronchial lung biopsy showed organised granulation tissue consisting of myofibroblasts and inflammatory cells within the alveolar space (Figure S1). Their clinical findings are shown in Table I. The mean age at the time of diagnosis of OP was 73 years. Fever was observed in all cases at the onset of OP. The median (range) time to onset of OP after MDS diagnosis was 8·5 (0·5–17) months. All patients were categorised as high-risk MDS. Interestingly cytogenetic studies revealed that three out of the five patients had a der(1;7)(q10; p10) abnormality. Subsequently we investigated the association between OP and karyotype der(1;7)(q10;p10). Among the 159 patients with MDS analysed, 11 (6·9%) harboured der(1;7)(q10;p10). Within this group of patients who harboured der(1;7)(q10;p10), three developed OP. The frequency of OP development in patients with MDS with der(1;7)(q10;p10) was 27·3% (three out of 11 patients). In contrast, only two of the 148 patients (1·4%) without der(1;7)(q10;p10) developed OP. The frequency of OP among the patients with der(1;7)(q10;p10) was higher than that among patients without der(1;7)(q10;p10) (odds ratio of 25·70: 95% confidence interval 2·582–347·4). These findings suggest that there is a relationship between MDS with der(1;7)(q10;p10) and OP. Regarding the treatments of MDS, two patients received azacitidine prior to the development of OP. The effect of azacitidine on MDS was that it stabilised the disease condition. After the onset of OP, both patients received only supportive care for MDS. The other three patients received only supportive care for MDS during the course. Treatment with prednisolone (PSL) was initiated against OP in all patients. Although PSL improved the symptoms transiently, there was lethal OP relapse in all cases after the reduction of PSL. All the patients with OP in the present study died from the progression of lung disease. The overall survival (OS) of patients with MDS after diagnosis with OP was 5·5 months. The patients with der(1;7) (q10;p10) who developed OP tended to have a shorter median of OS compared to those with the mutation harbouring no OP, but the difference was not statistically significant (20 vs. 38 months P = 0·931) (Figure S2).
| Patient number | Age, years | Gender | MDS subtype (WHO 2017 classification) | Karyotype | IPSS-R | Treatment of OP | Cause of death | Time to onset of OP after MDS diagnosis, months | OS after the onset of OP, months |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | Male | MDS-EB-1 |
46,XY,+1,der(1;7)(q10;p10) [19] 46,XY [1] |
High | PSL (0.5 mg/kg) | OP | 5 | 10 |
| 2 | 69 | Male | MDS-MLD |
46,XY,+1,der(1;7)(q10;p10) [15] 46,XY [5] |
High | PSL (0.5 mg/kg) | OP | 14 | 5·5 |
| 3 | 75 | Male | MDS-MLD |
46,XY,+1,der(1;7)(q10;p10) [12] 46,XY [8] |
Intermediate | PSL (0.6 mg/kg) | OP | 17 | 21 |
| 4 | 79 | Female | MDS-MLD |
50,XX,+1,del(5)(q22q35),+11,+14,del(20)(q11.2q13·1),+22 [10] 51,idem,+i(8)(q10) [3] |
High | PSL (0.8 mg/kg) | OP | 0·5 | 3 |
| 5 | 73 | Male | MDS-EB-2 | 46,XY [20] | High | PSL (0.5 mg/kg) | OP | 8·5 | 5 |
- MDS, myelodysplastic syndrome; MDS-EB, MDS with excess blast; MDS-MLD, MDS with multilineage dysplasia; IPSS-R, revised International Prognostic Scoring System; OP, organising pneumonia; PSL, prednisolone.
The mean annual incidence of OP was reported to be ~0·002% (1·97 out of 100 000 population).3 Previously Nanah et al.4 analysed 827 patients with MDS. They reported that four patients had OP and the frequency of OP in MDS was 0·5%. In the present study the incidence of OP in MDS was 3·1%. The results suggest that patients with MDS are more likely to develop OP compared to the general population. Furthermore, our present study detected a high frequency of OP in MDS with der(1;7)(q10;p10) in comparison with the frequency in MDS without the mutation. Five cases of der(1;7)(q10;p10)-harbouring MDS with the complication of OP have been reported to date.5-9 Further study is required to confirm if der(1;7)(q10;p10) is a risk factor for developing OP in MDS.
There are a few proposed theories on the relationship between MDS and OP. There is a prevalence of autoimmune disorders among patients with MDS of around 10–30% and the deregulation of innate immune and inflammatory signalling is described.10 As OP occurs in the context of various autoimmune or inflammatory disorders,2 these conditions may exist in the background of OP in MDS. We also assume genetic abnormality including der(1;7)(q10;p10) is related to the development of OP. The mutation of GATA2 was detected in 7% of children and adolescents MDS.11 Interestingly, one of the most common abnormal karyotypes associated with mutated GATA2-related MDS is der(1;7)(q10;p10). Furthermore, the phenotypic spectrum of the germline GATA2 mutations can show pulmonary diseases including OP.12 In addition, Okuda et al.13 recently analysed the molecular features of 84 cases of der(1;7)(q10;p10)-harbouring MDS and demonstrated that GATA2 was mutated in ~10% of cases. It is worth examining the mutation of GATA2 in der(1;7)(q10;p10)-positive MDS with OP.
The standard treatment of OP is corticosteroids, and the prognosis of idiopathic OP is usually not poor; however the mortality of secondary OP is higher than idiopathic OP.14 All the patients with OP in this study had a limited effect of PSL. There are previous case reports of treating two patients with OP harbouring der(1;7)-positive MDS with hypomethylating agents (HMA) in addition to PSL. HMA was effective for OP regardless of its effect on MDS.7 The effects of azacitidine in autoimmune disorders associated with MDS have been reported.15 We can also expect the effects of azacitidine on OP associated with MDS. One patient with MDS with OP harbouring der(1;7)(q10;p10) received allogeneic haematopoietic stem cell transplantation (HSCT).9 The patient achieved cytogenetic remission after HSCT and then OP gradually improved. Thus, HSCT a curative treatment for MDS, may also be effective on its complication of OP.
In conclusion, our study results imply a possible relationship between OP and der(1;7)(q10; p10) in MDS. Further study with a larger number of cases shall validate our hypothesis and unravel the mutation’s pathogenesis and the impact on MDS clinical features.
Acknowledgements
We give special thanks to Ayako Komoto for her excellent editorial support to the authors during the preparation of this manuscript.
Author contributions
Manabu Matsunawa conducted the study, analysed the data and wrote the manuscript. Ayako Arai analysed the data and wrote the manuscript. Yasuyuki Inoue, Akiko Uchida, Yu Uemura, Yusuke Saiki, Madoka Takimoto and Fumiaki Sano treated the patients and analysed the data. Kunihiro Yagihashi analysed the radiological findings. Yoshio Aida analysed the pathological findings. Ikuo Miura analysed the data and reviewed the manuscript. All authors approved the final version of the manuscript.
Conflict of interest
The authors declare to have no potential conflicts of interest.