Insights into male androgenetic alopecia using comparative transcriptome profiling: hypoxia-inducible factor-1 and Wnt/β-catenin signalling pathways*
Q.L., Y.T., Y.H. and Ji’an Wang contributed equally.
Plain language summary available online
Abstract
Background
The key pathophysiological changes in androgenetic alopecia (AGA) are limited to hair follicles (HFs) in frontal and vertex regions, sparing the occipital region.
Objectives
To identify biological differences among HF subpopulations.
Methods
Paired vertex and occipital HFs from 10 male donors with AGA were collected for RNA sequencing assay. Furthermore, HF and cell experiments were conducted on the identified key genes to reveal their roles in AGA.
Results
Transcriptome profiles revealed that 506 mRNAs, 55 microRNAs and 127 long noncoding RNAs were differentially expressed in the AGA vertex HFs. Pathway analysis of mRNAs and microRNAs revealed involvement of the hypoxia-inducible factor (HIF)-1, Wnt/β-catenin, and focal adhesion pathways. Differential expression of HIF-1 prolyl hydroxylase enzymes (EGLN1, EGLN3) and Wnt/β-catenin pathway inhibitors (SERPINF1, SFRP2) was experimentally validated. In vitro studies revealed that reduction of EGLN1, EGLN3, SERPINF1 and SFRP2 stimulated proliferation of dermal papilla cells. Ex vivo HF studies showed that downregulation of EGLN1, EGLN3 and SERPINF1 promoted HF growth, postponed HF catagen transition, and prolonged the anagen stage, suggesting that these genes may be potentially utilized as therapeutic targets for AGA.
Conclusions
We characterized key transcriptome changes in male AGA HFs, and found that HIF-1 pathway-related genes (EGLN1, EGLN3) and Wnt pathway inhibitors (SERPINF1, SFRP2) may play important roles in AGA.
- Multiple differentially expressed genes and signalling pathways have been found between hair follicles (HFs) in the balding area (frontal and vertex regions) and nonbalding area (occipital region) of individuals with androgenetic alopecia (AGA).
- A whole-transcriptome atlas of the vertex and occipital region is lacking.
- We identified a number of differentially expressed genes and pathways between balding vertex and nonbalding occipital AGA HFs by using whole-transcriptome analyses.
- We identified pathways not previously reported in AGA, such as the hypoxia-inducible factor (HIF)-1 signalling pathway.
- We verified that HIF-1 pathway-related genes (EGLN1, EGLN3) and Wnt pathway inhibitors (PEDF, SFRP2) played important roles in dermal papilla cell activity, hair growth and the hair cycle.
- The EGLN1, EGLN3, SERPINF1 and SFRP2 genes may be potentially utilized as therapeutic targets for AGA.
Androgen alopecia (AGA), or male pattern hair loss, is the most common type of progressive hair loss.1 It often becomes a psychological burden and affects the normal social life of affected individuals.2 Currently, minoxidil and finasteride are the only two drugs that have obtained approval from the US Food and Drug Administration (FDA) for AGA treatment. In addition to a scarcity of pharmacological approaches, alopecia can also be treated with various hair loss strategies, including hair transplants and germinal caps.
In normal adults, hair follicles (HFs) undergo a lifelong cyclical transformation, progressing through stages of rapid growth (anagen), regression (catagen) and relative quiescence (telogen).3 However, in AGA, due to the effect of the testosterone metabolite dihydrotestosterone on susceptible HFs, the anagen phase of HFs is gradually reduced while the telogen phase is prolonged.4, 5 This change results in HF miniaturization,6 leading to a transformation from terminal to vellus-like hair in individuals with AGA. As the acknowledged target, dermal papillae (DPs) are the main sites of androgen activity in HFs.7, 8 The miniaturization seen in the patterned hair loss may be the direct result of the reduction in the number of dermal papilla cells (DPCs) and, hence, in the size of the DPs.
In male AGA, hair loss typically involves the temporal and vertex region, generally sparing the occipital region.9 Several gene expression profiling studies have identified differences in the expression levels of genes and signalling pathways between HFs in the balding area (i.e. frontal and vertex regions) and nonbalding area (i.e. occipital region) of individuals with AGA,10, 11 including androgen metabolism, the Wnt/β-catenin pathway, and redox state.12-18 However, these studies only detected the mRNA alterations while the changes of other noncoding RNAs were neglected. Moreover, previous transcriptome studies mainly focused on plucked HFs, resulting in damage of key cell types in AGA, including DPCs and the bulge region cells.19
To further explore the pathogenesis of AGA, we performed a whole-transcriptome analysis to identify the differentially expressed mRNAs, microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) in HF subpopulations. The HFs in balding vs. nonbalding scalp were obtained by follicular unit extraction so they would consist of more key cell types from the same male patients with AGA in our study. Furthermore, we conducted follow-up in vitro and ex vivo experiments to verify and reveal the roles of identified genes and pathways in AGA pathogenesis.
Materials and methods
Human hair follicle organ dissection
All male participants with AGA signed informed consent and were notified about the study before participation. The study was approved by Huashan Hospital of Fudan University (ethical approval number 2019M-008). The clinical information of the study participants is shown in Table S1 (see Supporting Information). Samples from patients 1–10 were used for RNA sequencing and those from patients 1–20 were used for the follow-up functional studies. Anagen HFs in the vertex and occipital areas from male patients with AGA undergoing hair transplants were collected by follicular unit extraction, and haematoxylin and eosin staining was performed to confirm the hair cycle stage (Figure S1; see Supporting Information). Anagen follicles were used in this study and were used in RNA sequencing analysis and ex vivo HF studies.
RNA sequencing and bioinformatic analysis
The parameters, reagents and methods for sequencing and bioinformatic analysis can be found in Appendix S1 (see Supporting Information).20-28
Human hair follicle culture and treatment
The isolated human HFs were cultured in Williams E medium (Gibco BRL, Grand Island, NY, USA). HFs that grew to lengths of 0·3–0·5 mm and macroscopically remained in anagen VI after 24 h were selected for further experiments.29 For transfection experiments, HFs were transfected with 0·04 nmol L−1 EGLN1, EGLN3, SFRP2 or SERPINF1 (formerly PEDF) small interfering (si)RNA mixed with 2 μmol L−1 Lipofectamine RNAiMAX transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA). The transfection effect was evaluated at 2 days post-transfection by immunofluorescence. For recombinant protein stimulation experiments, HFs were incubated with 100 ng mL−1 recombinant pigment epithelium-derived factor (PEDF) protein (HY-P7054; MedChemExpress, Monmouth Junction, NJ, USA) or 1 μ1 recombinant secreted Frizzled-related protein 2 (SFRP2) protein (6838-FR-025; R&D Systems Inc., Minneapolis, MN, USA). The length of the hair shaft and the hair cycle were measured every 2 days.
Immunofluorescence staining
HF samples were embedded in paraffin. Next, histological sections were incubated overnight at 4 °C with primary antibodies for either EGLN1 (1 : 300, sc-271835; Santa Cruz Biotechnology, Santa Cruz, CA, USA), EGLN3 (1 : 250, ab184714; Abcam, Cambridge, UK), PEDF (1 : 500, ab180711; Abcam) or SFPR2 (1 : 500, ab92667; Abcam). The sections were then incubated with the corresponding secondary antibody for 1 h at room temperature. After removing the secondary antibody, the nucleus was counterstained with 4-amino-6-diamino-2-phenylindole (DAPI; Beyotime Biotech, Nanjing, China). The stained sections were observed under a microscope (Olympus, Tokyo, Japan), and representative images were captured.
Real-time polymerase chain reaction
DPCs were isolated from HF bulbs and cultured in Dulbecco’s modified Eagle’s medium as previously reported.30 Total RNA was extracted and reverse transcribed from HFs or DPCs using TRIzol (Invitrogen, Carlsbad, CA, USA) and the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Real-time polymerase chain reaction (PCR) was performed with SYBR Premix Ex Taq (Takara Bio, Shiga, Japan) and analysed with an ABI Prism 7900 Detector System (Applied Biosystems). The housekeeping gene GAPDH was used as the endogenous control.
Real-time cellular analysis assay
Real-time cellular analysis (Agilent, Santa Clara, CA, USA) was applied to monitor cell viability according to the manufacturer’s instructions. An xCELLigence system (Roche Applied Science, Penzberg, Germany) was used to record the cell growth curves automatically. Cell index was used to observe cell state (e.g. cell attachment, cell number). Cells at a density of 9000 cells per well were seeded in a 16-well microplate. After incubation for 6 h, the medium was refreshed with new treatment medium.
Western blot
Cell lysates were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and proteins were transferred onto polyvinylidene fluoride (PVDF) membranes. According to the standard protocol, the PVDF membranes were blocked in Tris-buffered saline with Tween (TBST, pH 7·4) and 5% bovine serum albumin for 2 h and incubated with their respective primary antibodies for 8 h at 4 °C, followed by the secondary antibody for 2 h at room temperature. After washing with TBST three times, the proteins were visualized using the ECL chemiluminescence system (Santa Cruz Biotechnology). ImageQuant TL software (Cytiva, Marlborough, MA, USA) was used to quantify the intensity of bands.
Deferoxamine treatment
Deferoxamine was purchased from Sigma-Aldrich (St Louis, MO, USA). The working concentration for treatment of DPCs was 300 ng mL−1. RNA and protein were extracted from DPCs after 24-h and 48-h incubation periods with or without deferoxamine, respectively.
Statistical analysis
All data were analysed using R (v3.6.0; R Foundation, Vienna, Austria). The paired-sample t-test and unpaired-sample t-test were used for paired samples and unpaired samples, respectively. P-values < 0·05 following false discovery rate adjustment for multiple testing correction were considered to be statistically significant.
Results
Whole-transcriptome signatures of hair follicles from individuals with androgenetic alopecia
To explore the pathogenesis of AGA, we performed RNA sequencing of protein-coding genes, lncRNAs and miRNAs in paired HFs of the frontal vertex and occipital scalp from 10 male patients with AGA (Figure S1). The whole-genome transcriptomic profiles of these two groups were pooled together and presented as a circle plot based on their P-values and the chromosome location of each gene (Figure 1a). Differential expression analysis identified 506 mRNAs, 127 lncRNAs and 55 miRNAs that were differentially expressed. Hierarchical clustering indicated that most mRNAs and miRNAs were upregulated in the vertex group compared with the occipital group (Figure 1b, c), whereas lncRNAs were mainly downregulated (Figure S2a; see Supporting Information). For the differentially expressed mRNAs, 308 genes were upregulated, and 198 downregulated genes were identified in the vertex group (Figure 1d). In miRNAs, we identified 35 upregulated miRNAs and 20 downregulated miRNAs (Figure 1e). We also detected 53 upregulated lncRNAs and 74 downregulated lncRNAs (Figure S2b).
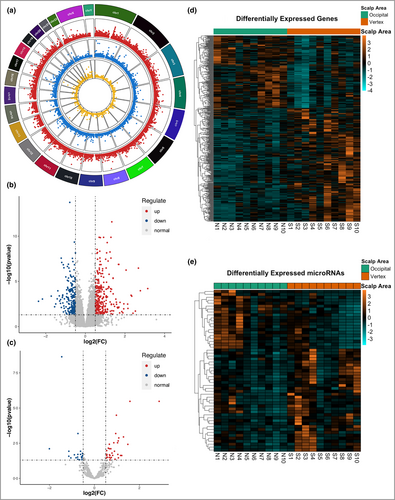
These results showed significantly differential expression patterns between vertex and occipital HFs in AGA. The details of the differentially expressed genes, miRNAs and lncRNAs are listed in Tables S2–4 (see Supporting Information). In addition, we identified the correlation pairs of differentially expressed RNAs through correlation analysis and constructed regulatory networks using these correlation pairs. The lncRNA–mRNA coexpression networks and miRNA–mRNA and miRNA–lncRNA regulatory networks were constructed based on their coefficients using Cytoscape28 (Figure S3a–c; see Supporting Information). Moreover, a competitive endogenous RNA network was constructed to identify the key regulators using miRNA–mRNA and miRNA–lncRNA pairs (Figure S3d). The results of the coexpression analysis are presented in Tables S5–7 (see Supporting Information).
Pathway enrichment analysis: hypoxia-inducible factor-1 and Wnt signalling pathways
We further performed KEGG pathway enrichment analysis to identify the altered signalling pathways in AGA. We found that the upregulated mRNAs in AGA vertex HFs were significantly enriched in the HIF-1 signalling pathway (adjusted P = 9·8 × 10−7). The downregulated mRNAs were mainly enriched in the Wnt signalling pathway (adjusted P = 7·9 × 10−5) and Hippo signalling pathway (adjusted P = 8·1 × 10−5) (Figure 2a). The expression of key HIF-1 signalling genes (EGLN1, EGLN3, SERPINE1, HMOX1, TIMP1, and IL6R) and Wnt signalling-related genes (SERPINF1, SFRP2, DKK1, LGR4, LGR5, WNT3A, WNT5A and WNT16) is shown in Figure 2(b–d). Furthermore, Gene Ontology enrichment analysis revealed that adaptive immune response, moulting cycle, hair cycle, extracellular matrix, cytokine receptor activity, DNA-binding transcription activator activity, and hormone activity pathways were dysfunctional in balding HFs (Tables S8–10; see Supporting Information).
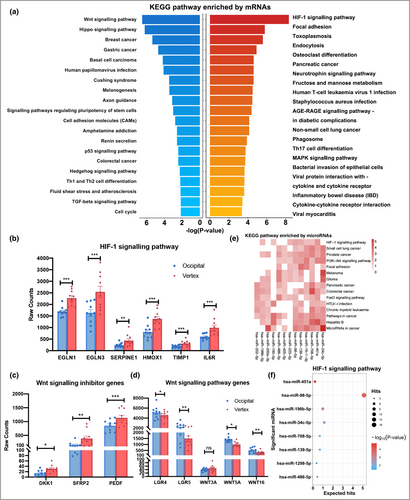
We also performed miRNA pathway enrichment analysis using miRPathDB.20, 23 We found that the HIF-1 and Wnt signalling pathways (data not shown) were significantly enriched with differentially expressed miRNAs using KEGG and WIKI pathway analysis, respectively (Figure 2e). In particular, the target genes of miR-451a, miR-98-5p and miR-196b-5p were significantly enriched in the HIF-1 signalling pathway (Figure 2f). In addition, the focal adhesion, cell cycle, DNA damage response and transforming growth factor-β signalling pathways were highly involved. These miRNA-enriched pathways were coordinated with mRNA-enriched pathways, which emphasizes the importance of the pathways that we identified. Therefore, the HIF-1 and Wnt signalling pathways were selected for further functional studies.
Expression validation of differentially expressed genes involved in the hypoxia-inducible factor-1 and Wnt signalling pathways
To validate the expression levels of differentially expressed genes, a total of 20 AGA samples, including 10 independent pairs of HFs, and the initial 10 pairs of HFs for RNA sequencing were collected (Table S1). Haematoxylin and eosin staining showed that the obtained HFs were anagen HFs, and revealed a concomitant decrease in the maximum bulb diameter of vertex HFs compared with occipital HFs (Figure 3a). Real-time PCR was performed to assess the expression of HIF-1 pathway-related genes (EGLN1, EGLN3 and HMOX1) and Wnt signalling pathway-related genes (SERPINF1, SFRP2 and LGR5). The results showed that the mRNA levels of EGLN1, EGLN3, SERPINF1 and SFRP2 were significantly higher in vertex HFs compared with occipital HFs (Figure 3b; and Figure S4a; see Supporting Information).
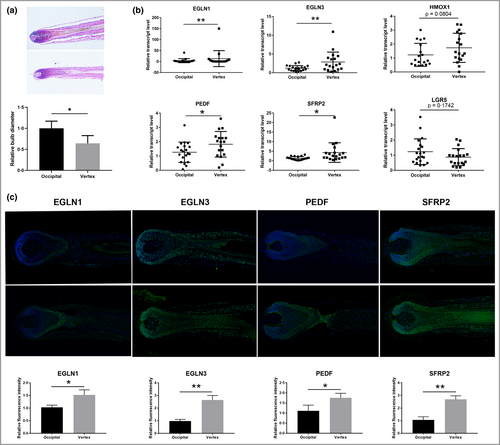
Moreover, immunofluorescence staining showed increased mean (SD) protein expression of EGLN1 [1·04 (0·24) vs. 1·55 (0·35)], EGLN3 [1·02 (0·06) vs. 2·65 (0·38)], SERPINF1 [1·15 (0·25) vs. 1·75 (0·18)] and SFRP2 [1·16 (0·21) vs. 2·54 (0·53)] in vertex HFs (Figure 3c). These proteins were ubiquitously expressed in almost every cell type, and EGLN1 was mainly expressed in hair matrix cells (Figure 3c). Collectively, we discovered that HIF-1 and Wnt signalling pathway-related genes (EGLN1, EGLN3, SERPINF1 and SFRP2) were upregulated in AGA vertex HFs and might play important roles in AGA.
The effects of hypoxia-inducible factor-1 pathway-related EGLN1 and EGLN3 on dermal papilla cells and hair follicles
DPC proliferation activity, HF growth and the hair cycle play important roles in the process of HF miniaturization and AGA. To assess the effects of HIF-1 pathway-related genes (EGLN1 and EGLN3) in AGA, we treated DPCs and cultured HFs with EGLN1 or EGLN3 siRNA. Real-time PCR analysis confirmed the decreased expression of EGLN1 or EGLN3 after corresponding siRNA transfection into cultured DPCs (Figure 4a). Cell proliferation activity was examined by the xCELLigence system as reported,31 and we found that downregulation of EGLN1 or EGLN3 resulted in a significant increase in DPC proliferation (Figure 4b). Furthermore, the decreased expression of EGLN1 and EGLN3 in treated HFs was confirmed by immunofluorescence (Figure 4c). As shown in Figure 4d, EGLN1 or EGLN3 knockdown increased the length of hair shafts compared with the negative control. Moreover, a greater percentage of anagen HFs and a lower percentage of catagen HFs remained in the EGLN1 or EGLN3 siRNA-treated group compared with the negative control group after 6 days of culture (Figure 4e). The results revealed that EGLN1 or EGLN3 downregulation promoted DPC proliferation and HF growth, prolonged the anagen stage, and postponed HF catagen transition, suggesting the important role of EGLN1 and EGLN3 in AGA.

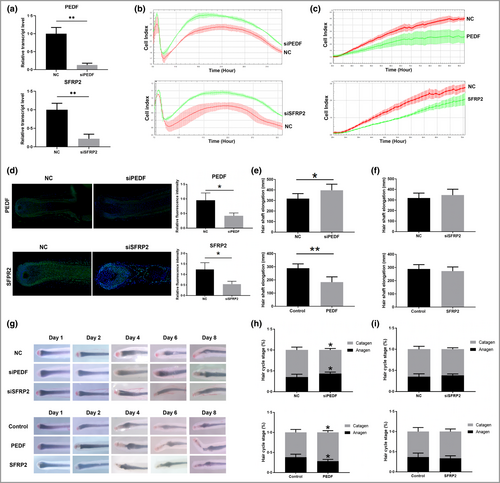
The effects of Wnt pathway-related SFRP2 and SERPINF1 on dermal papilla cells and hair follicles
The Wnt signalling pathway plays a key role in AGA, but the effect of SFRP2 or SERPINF1 has not been studied in AGA. Here, we show that SFRP2 or SERPINF1 siRNA treatment reduced the transcriptomic levels of targeted genes (Figure 5a) and significantly promoted DPC proliferation (Figure 5b). Consistently, addition of recombinant SFRP2 or SERPINF1 protein inhibited DPC proliferation (Figure 5c). In HFs, SFRP2 or SERPINF1 siRNA transfection decreased target protein expression as revealed by immunofluorescence (Figure 5d). In addition, SERPINF1 downregulation promoted HF growth, while the SERPINF1 recombinant protein significantly inhibited HF growth (Figure 5e). However, SFRP2 siRNA or recombinant protein treatment showed no effect on HF growth (Figure 5f). Compared with the negative control group, SERPINF1 downregulation resulted in a greater percentage of anagen HFs remaining and a lower percentage of catagen HFs, and SERPINF1 recombinant protein facilitated catagen transition and shortened the anagen stage (Figure 5g, h). Although SFRP2 is an inhibitor of the Wnt pathway, SFRP2 did not significantly affect the HF hair cycle (Figure 5g, i). These findings suggest that as SERPINF1 is an inhibitor of the Wnt pathway, its upregulation plays a role in HF pathological changes and the development of AGA.
Discussion
Dissecting the transcriptomic alterations between AGA-affected and AGA-unaffected HFs may significantly improve our understanding of AGA pathogenesis. Hochfeld et al. detected the transcriptomic changes between plucked HFs from the frontal and occipital scalp regions of patients with AGA, suggesting that the ephrin and Hippo signalling pathways may contribute to the deregulation of HF growth and HF miniaturization.19 In addition, multiple miRNAs, including has-miR-106a, has-miR-106b, has-miR-125b, has-miR-221 and has-miR-410, were found to be upregulated in the balding DPCs.32, 33 Moreover, hsa-miR-133b was dysregulated in the AGA scalp and could affect the proliferation of DPCs via the Wnt/β-catenin pathway.34 Also, has-miR-324-3p was depleted in balding follicular stem cells from patients with AGA and could affect the differentiation programme of keratinocytes.35 However, few studies have systemically identified the transcriptomic changes of AGA HFs at the whole-transcriptome level. In this study, we have identified differentially expressed mRNA, miRNAs and lncRNAs between the male AGA HFs of the occipital and vertex regions, and highlighted the roles of the HIF-1 and Wnt/β-catenin signalling pathways in AGA pathogenesis.
The HIF-1 signalling pathway plays a crucial role in regulating cellular homeostasis under hypoxia. HIF-1 transactivates an array of gene-producing proteins capable of increasing oxygen supply, including vascular endothelial growth factor (VEGF), which promotes angiogenesis.36, 37 The HIF subunit is usually prolyl hydroxylated by EGLN (also called prolyl hydroxylase domain, PHD) family members under normoxic conditions, causing its rapid degradation. Previous studies have reported that hair loss, including AGA, is strongly associated with a reduced vascularization of HFs.38 Minoxidil, an FDA-approved drug for AGA treatment, may activate the HIF-1–VEGF pathway, and PHD may be the molecular target for minoxidil-mediated VEGF induction via HIF-1 in keratinocytes and DPCs.39 A combination of pyridine-2,4-dicarboxylic acid diethyl ester and resveratrol, which stabilizes HIF-1α and reduces oxygen peroxide-induced oxidative stress, improved hair density in females donors.40 Moreover, our study also found that downregulation of EGLN1 or EGLN3 could stimulate DPC proliferation and promote HF growth. Therefore, we suggest that EGLN1 or EGLN3 may regulate DPC and HF growth through the HIF-1–VEGF pathway.
To further verify this assumption, we first confirmed the decreased expression and protein levels of HIF-1α and VEGFA in the vertex HFs of patients with AGA (Figure S4b, c), and found they could be restored after siEGLN1 or siEGLN3 treatment (Figure S4d). In accordance, expression and protein levels of HIF-1α and VEGFA could be upregulated after siEGLN1 or siEGLN3 treatment in DP cells (Figure S5a–d; see Supporting Information). Furthermore, DP cells treated with deferoxamine (an iron chelator) could also upregulate transcriptomic and protein levels of HIF-1α and VEGFA, resulting in a higher proliferation of DP cells (Figure S5e–g). Taking the results together, we suggest that EGLN1 and EGLN3 may regulate HF DPs and HFs through the HIF-1–VEGF signalling pathway. However, a previous study found that loss of EGLN1 could activate the HIF-dependent hypoxic response, including anaerobic glycolysis,41 suggesting that EGLN1 and EGLN3 might regulate HF metabolism to participate in AGA pathogenesis. In addition, we only examined the role of EGLN1 and EGLN3 in DP cells, and the effect of EGLN1 and EGLN3 on HF epithelial cells remains elusive. Further investigations are required to clarify the diverse effects of EGLN1 and EGLN3 on different compartments of HFs.
Wnt/β-catenin signalling plays a pivotal role in HF morphogenesis, development and regeneration during the hair cycle.12 In accordance with previous studies, we found downregulation of the Wnt signalling pathway in the vertex HFs of patients with AGA compared with occipital HFs. Targeting Wnt signalling is more likely to come from approaches that interfere with Wnt antagonists and restore signalling. Extensive research has shown that modulation of Wnt natural inhibitors, including DKK1, DKK2, SFRP1 and SFRP4, could result in comparable growth-promoting effects while alleviating some safety concerns.15, 18, 42-44
In this study, we scanned the common Wnt signalling pathway inhibitor genes and found that only four of these genes (SFRP2, SERPINF1, DKK1 and IGFBP3) were significantly upregulated in the AGA vertex region, and few studies have focused on the roles of SFRP2 and PEDF in AGA. Therefore, we further examined these two Wnt signalling pathway inhibitors for follow-up experiments. We found that SERPINF1 downregulation stimulated DPC proliferation, promoted HF growth, postponed HF catagen transition, and prolonged the anagen stage, while PEDF recombinant protein showed opposite results. SFRP2 has been widely recognized as an inhibitor of Wnt signalling, and SFRP2 downregulation can activate Wnt siganling.45-47 However, Kwack et al. reported that SFRP2 could augment WNT3a-mediated Wnt signalling, suggesting that the role of SFRP2 in the Wnt signalling pathway is complex and warrants further clarification.48 In our study, we found that exogenous SFRP2 could inhibit DPC proliferation. However, treatment with SFRP2 siRNA or recombinant protein showed no significant effects on HF growth, which might be due to the effects of functional SFRP1 as previously reported.18
In conclusion, our study characterizes the whole-transcriptome signatures of HFs from the balding vertex and nonbalding occipital scalp areas of patients with AGA, highlighting the HIF-1 signalling pathway and Wnt/β-catenin pathway in AGA pathogenesis. We identified that HIF-1 pathway-related genes (EGLN1 and EGLN3) and Wnt/β-catenin pathway inhibitors (SFRP2 and SERPINF1) may play important roles in DPC activity, hair growth and the hair cycle, and may potentially be utilized as targets for AGA and hair loss treatment. Further studies with larger sample sizes are awaited to validate these findings in the future. We anticipate that our data will serve as a valuable resource facilitating future studies for patients with AGA.
Acknowledgments
We thank all of the authors for their efforts in this work and all of the participants involved in this study. Wenyu Wu, Jinran Lin and Jiucun Wang contributed equally to this work.
Author contributions
Qingmei Liu: Methodology (lead); project administration (equal); writing – original draft (lead). Yulong Tang: Data curation (lead); software (lead); writing – original draft (lead). Yan Huang: Methodology (lead); validation (lead); writing – original draft (equal). Ji’an Wang: Methodology (lead); validation (lead). Kai Yang: Investigation (equal); resources (equal). Yuting Zhang: Methodology (equal); validation (equal). Weilin Pu: Methodology (equal); software (equal). Jing Liu: Software (equal); validation (equal). Xiangguang Shi: Methodology (equal); validation (equal). Yanyun Ma: Methodology (equal); software (equal). Chunya Ni: Resources (equal); writing – original draft (equal). Yue Zhang: Investigation (equal); methodology (equal). Yifei Zhu: Methodology (equal); visualization (equal). Haiyang Li: Software (equal); validation (equal). Jiucun Wang: Project administration (lead); supervision (lead). Jinran Lin: Project administration (lead); supervision (lead). Wenyu Wu: Project administration (lead); supervision (lead); writing – original draft (lead); writing – review and editing (lead).
Funding sources
This study was supported by grants from National Science Foundation of China (82173442, 82103759), Shanghai Engineering Research Center of Hair Medicine (19DZ2250500), Clinical Research Plan of SHDC (SHDC2020CR2033B), Science and Technology Committee of Shanghai Municipality Guiding Fund (19411962400), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-066), the innovative research team of high-level local universities in Shanghai, and Shanghai Municipal Science and Technology Major Project (2017SHZDZX01).
Conflicts of interest
The authors declare they have no conflicts of interest.
Ethics statement
The study was approved by Huashan Hospital of Fudan University (ethical approval number 2019M-008).
Open Research
Data availability
The data and the code that support the findings of this study are available on reasonable request from the corresponding author.




