Traditional Chinese Medicine for Inhibiting Ferroptosis in Ischemic-Related Diseases
Funding: This work was supported by the National Natural Science Foundation of China (82304493), the Science and Technology Research Project of Chongqing Education Commission (KJZD-M202302701), the Wanzhou District Social Welfare Research Project (202206151527122088), the Proof of Concept Project of Medical Innovation Center (Taizhou) of Peking University (CXYZ-2024-101), and the Key Laboratory Special Project of Chongqing Three Gorges Medical College (Sys20210002).
ABSTRACT
Ischemic-related diseases, such as myocardial infarction and stroke, are primarily driven by a deficit in oxygen supply leading to cellular damage and death. Ferroptosis has emerged as an important mechanism contributing to the progression of ischemic injury, characterized by iron-dependent lipid peroxidation. This review aims to provide a comprehensive overview of the significant mechanisms underlying ferroptosis in ischemic conditions and explores the potential effects of traditional Chinese medicines (TCMs) and their extracts. Numerous compounds extracted from TCMs, including flavonoids, polyphenols and terpenes, exhibit potent antiferroptotic effects by activating nuclear factor erythroid 2–related factor 2, upregulating glutathione peroxidase 4, inhibiting lipid peroxidation and so on. These properties render TCMs a promising candidate for developing novel ferroptosis therapeutic strategies. This review underscores the importance of investigating the interactions between ferroptosis and TCMs within the context of ischemic diseases. These findings provide valuable insights for future research to identify targets associated with ferroptosis regulation, thereby expanding the pharmacological perspective of TCMs in treating ischemic diseases and revealing the potential of novel therapeutic strategies. Additionally, this highlights the relevance of integrating traditional and modern medical approaches in addressing complex health issues.
Summary
- This review discusses how ferroptosis works in ischemic conditions and the potential benefits of traditional Chinese medicines (TCMs) and their extracts.
- Many TCMs' compounds, such as flavonoids and polyphenols, can help prevent ferroptosis by enhancing protective enzymes and reducing lipid damage.
- The review emphasizes the need to study how ferroptosis and TCMs interact, offering insights for future research on targets related to ferroptosis and improving TCMs' role in treating these conditions.
- It also highlights the importance of combining traditional and modern medical approaches to tackle complex health issues.
1 Introduction
Ischemic-related disease is a variety of pathological states caused by insufficient blood supply to tissues or organs, characterized by local hypoxia, malnutrition and accumulation of metabolic products. Such diseases are usually closely related to factors such as vascular stenosis, embolism and arteriosclerosis. The main diseases caused by them include ischemic heart disease, ischemic stroke and peripheral arterial disease. The common feature of these diseases is restricted blood flow, leading to corresponding organ dysfunction and, in severe cases, even causing tissue necrosis and death. Data from the World Health Organization show that cardiovascular disease is the deadliest disease worldwide, accounting for approximately 32% of total deaths. Among them, ischemic heart disease accounts for nearly half of cardiovascular disease deaths (https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)). However, there are still many challenges in the current treatment methods for ischemic-related diseases, and commonly used drugs such as antiplatelet drugs, anticoagulant drugs and reperfusion therapy cannot achieve ideal results for some patients [1]. Accompanying bleeding risks, drug resistance and other issues in clinical application limit the effectiveness. Therefore, it is imperative to delve into the mechanisms of occurrence and development of ischemic-related diseases and their therapeutic targets.
The initial phases of ischemia trigger a cascade of pathological events, including inflammation, oxidative stress and metabolic alterations [2]. During reperfusion, the restoration of blood flow, reactive oxygen species (ROS) is significantly elevated, which exacerbates cellular damage. A recently recognized mechanism contributing to cell death in ischemic conditions is ferroptosis, a form of regulated cell death characterized by the accumulation of lipid peroxides. Unlike traditional forms of cell death, ferroptosis is driven by iron-dependent oxidative processes that disrupt the cell's redox balance [3]. This process is prominently involved in various ischemic diseases, where the excessive buildup of lipid peroxides leads to cellular injury and death. Iron-induced cell death may further exacerbate cellular damage by participating in various pathways such as microenvironment regulation, cellular metabolism and inflammatory response [4]. Therefore, exploring the role of ferroptosis in ischemic diseases has important clinical translational value.
Traditional Chinese medicine (TCM) is the treasure of China, and it cares for human health. It has demonstrated good efficacy and minimal side effects in treating various diseases. There is also an increasing amount of research on its ability to alleviate ferroptosis. Therefore, this review aims to review the effect of TCM on inhibiting ferroptosis in ischemic diseases, explore its potential mechanisms and related targets and provide clues and ideas for the discovery of therapeutic medicine or targets related to ferroptosis in the future.
2 Concept and Common Pathways in Ferroptosis
Ferroptosis is a novel form of cell death characterized by dependence on intracellular iron and accumulation of lipid peroxides. In 2012, Dixon and Olzmann [5] first defined and described this phenomenon. The occurrence of ferroptosis is mainly related to the excessive accumulation of intracellular iron ions, weakened antioxidant capacity and dysregulation of lipid metabolism [6] (Figure 1).
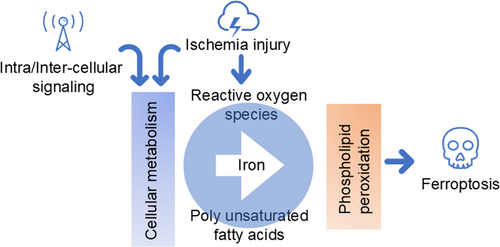
Oxidative stress caused by ischemia can lead to an increase in ROS, which in turn induces the accumulation of lipid peroxides. Second, under ischemic conditions, there is an imbalance in intracellular iron metabolism, accumulation of iron ions, promotion of lipid peroxidation and an increased tendency towards iron death. In addition, ischemia-related depletion of intracellular glutathione (GSH) can also reduce resistance to oxidative stress and promote the occurrence of ferroptosis. In addition, during ischemia–reperfusion, the damage to the cell membrane intensifies the uptake of iron by cells, while inhibiting antioxidant pathways such as reduced activity of thioredoxin 4, further promoting the development of ferroptosis.
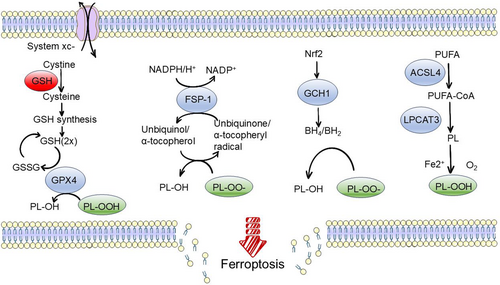
Classical ferroptosis involves both glutathione peroxidase 4 (GPX4)-regulated and GPX4-independent pathways [7]. GPX4, a selenoprotein, is crucial for reducing phospholipid hydroperoxides (PLOOHs) in mammalian cells. The canonical path involves cystine uptake via the system xc− antiporter, its reduction to cysteine, glutathione (GSH) biosynthesis and GPX4-mediated reduction of PL-OOH to corresponding alcohols (PL-OH). Oxidized glutathione (GSSG) recycling occurs through glutathione-disulphide reductase (GSR) with electrons from NADPH/H+. Independently, the FSP1/ubiquinone (CoQ10) system, by reducing ubiquinol/α-tocopherol, prevents lipid peroxidation and ferroptosis. Squalene and dihydrobiopterin/tetrahydrobiopterin (BH2/BH4) also inhibit lipid peroxidation and ferroptosis.
Uncontrolled lipid peroxidation is a hallmark of ferroptosis, initiated by the removal of a hydrogen atom from the polyunsaturated fatty acyl portion of phospholipids (PUFA-PL), leading to carbon-centred free radicals that react with molecular oxygen to produce peroxide free radicals. These radicals damage membrane integrity, destroying organelles and cell membranes. The unsaturation of the lipid bilayer is a critical determinant of cellular sensitivity to ferroptosis. Some lipoxygenases (LOX), which are nonheme iron-dependent dioxygenases, can directly oxygenate PUFA in biological membranes, facilitating the process of ferroptosis. Additionally, metabolic processes such as adipogenesis, autophagy and mitochondrial oxidation contribute to the production of PLOOHs that induce ferroptosis.
Moreover, iron plays a central role in cellular viability and death. Iron is crucial for various metabolic enzymes involved in generating cellular ROS. Furthermore, nonenzymatic, iron-dependent Fenton chain reactions are vital for ferroptosis. When GPX4 is inhibited, PLOOHs can persist longer, triggering Fenton reactions that rapidly amplify PLOOHs, which can react with ferrous and ferric ions to generate free radicals PLO• and PLOO•. These free radicals react with PUFA-PLs to propagate PLOOH production (Figure 2).
3 Methods
A comprehensive literature search was conducted to gather relevant studies on the role of TCMs in inhibiting ferroptosis and alleviating ischemic diseases. Appropriate keywords and phrases were selected, including ‘traditional Chinese medicine’, ‘ferroptosis’, ‘ischemic disease’, ‘antioxidant properties’ and ‘iron homeostasis’. Databases including Pubmed, Web of Science and Scopus are utilized to ensure the broad coverage of literature. Studies were filtered based on relevance to TCMs and ferroptosis in ischemic diseases. Only peer-reviewed articles, clinical trials and significant preclinical studies published in English were included. Reviews and articles with insufficient data were excluded. Relevant information was extracted from selected studies, focusing on ① types of TCMs and their specific components; ② experimental models (in vitro and in vivo); ③ mechanistic insights related to ferroptosis; and ④ clinical outcomes and safety profiles.
4 Universal Pathways of TCMs Against Ferroptosis
GPX4, solute carrier family 7 member 11(SCL7A11) and Acyl CoA synthase 4 (ACSL4) are considered to be the most reported targets of TCMs in alleviating ferroptosis. GPX4 is a vital antioxidant enzyme that reduces lipid peroxides in cellular membranes. By catalysing the reduction of lipid peroxides to their corresponding alcohols, GPX4 effectively safeguards cellular integrity from oxidative damage. Enhancing GPX4 activity can suppress ferroptotic cell death, highlighting it as a promising target for TCM interventions. On the other hand, SC7A11 is responsible for the import of cysteine, a critical precursor for glutathione synthesis. The upregulation of SLC7A11 can enhance cysteine uptake, thereby promoting GSH synthesis and reinforcing cellular defences against oxidative stress. In addition, ACSL4 catalyses the conversion of long-chain fatty acids into acyl-CoA, which contributes to the generation of phospholipid precursors involved in membrane synthesis and energy metabolism. The accumulation of certain fatty acid species, particularly PUFAs, promotes lipid peroxidation. SLC7A11 enhances the activity of GPX4 by increasing the supply of intracellular cysteine, promoting the synthesis of GSH and thus resisting oxidative stress. Meanwhile, the expression of ACSL4 plays a role in promoting oxidative stress in lipid metabolism. The regulatory relationship between these three constitutes the regulatory network of cells in the face of oxidative stress and iron death, revealing potential therapeutic targets in ischemia-related disease. In many original articles, these proteins are considered to be the key treatment targets of TCMs. However, it is not clear how they interact with TCMs or their extracts.
5 Organ-Specific Differences and Target Strategies of TCMs
5.1 TCMs Alleviate Ischemic Stroke and Transient Ischemic Attack via Inhibiting Ferroptosis
Stroke is a leading cause of death and disability worldwide, with the majority of cases resulting from ischemic events due to arterial occlusion. Current treatment strategies focus on rapid reperfusion through intravenous thrombolysis and endovascular thrombectomy. Iron metabolism is tightly regulated in the brain and is crucial for functioning. Imbalance of iron metabolism, including overload and deficiency, has been shown to impact the outcomes of ischemic stroke [8, 9]. Typical characteristics of ferroptosis, including lipid peroxidation and iron accumulation, are observed after ischemic stroke, accompanied by changes in the expression of ferroptosis-related genes such as GPX4, ACSL4 and SLC7A11 [9]. ACSL4 determines sensitivity to ferroptosis and may exacerbate ischemic stroke by increasing brain damage and neuroinflammation, targeting the prevention of neuronal loss [10, 11]. Furthermore, pathways relating to ferroptosis, oxidative stress and endoplasmic reticulum stress may share interactions or overlap [12].
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a key target inhibiting ferroptosis following ischemic stroke [13, 14]. Numerous monomers from TCMs have been confirmed to mitigate ischemic brain injury through activating Nrf2. Reports indicated that β-caryophyllene enhances Nrf2 nuclear translocation, reducing ROS production and ferroptosis induced by oxygen–glucose deprivation and reoxygenation (OGD/R) in primary astrocytes [15]. Vitexin has been shown to decrease the rate of Nrf2 translocation from the nucleus to the cytoplasm and regulate the Keap1/Nrf2/heme oxygenase (HO)-1 signalling pathway to suppress ferroptosis [16]. Quercetin can enter the cytoplasm, chelate iron and reduce intracellular unstable iron pools [17], thereby inhibiting oxidative stress and protecting the integrity of the blood–brain barrier (BBB) [18]. A recent study by Peng et al. [19] demonstrated that quercetin improves neurological functions, reduces infarct volume and pathological features, decreases lipid peroxide accumulation and suppresses ferroptosis by regulating the Nrf2/HO-1 signalling pathway in middle cerebral artery occlusion (MCAO) rats. Rhein reduces brain infarct area and BBB damage by mediating the Nrf2/SLC7A11/GPX4 signalling pathway, decreasing oxidative stress and ROS production, and inhibiting ferroptosis [20]. Gastrodin inhibits ferroptosis by regulating the Nrf2/Keap1/GPX4 signalling pathway, thus improving cognitive impairment in vascular dementia rats [21]. Loureirin C, purified from Dracaena cochinchinensis (Lour.) [22] and Icariside II, derived from the herbal medicine Herba Epimedii [23], promote Nrf2 nuclear translocation, activate the Nrf2/HO-1 pathway, upregulate GPX4, and reduce the accumulation of ROS and Fe2+ to improve neurological function and suppress ferroptosis in middle cerebral artery occlusion/reperfusion (MCAO/R) mice. Additionally, astragaloside IV inhibits ferroptosis by activating Nrf2 transcription and regulating the P62/Keap1/Nrf2 signalling pathway [24]. Leonurine A has been found to inhibit ferroptosis and exert neuroprotective effects by activating the phosphoinositide 3-kinase (PI3K)/AKT/Nrf2 and SLC7A11/GPX4 signalling pathways [25]. Enhancing GPX4 expression may be another important pathway to inhibit ferroptosis in ischemia-related diseases. Dihydrotanshinone I, a lipophilic danshen ketone extracted from Salvia miltiorrhiza, has been shown to reduce the accumulation of lipid peroxides and upregulate GPX4 protein expression in PC12 cells, thereby inhibiting ferroptosis. It also has been reported to reduce brain infarct area and improve ferroptosis by activating the Nrf2 pathway in MCAO/R rats [26]. Dihydromyricetin can reduce brain infarction in MCAO/R rats, restore neurological function, and inhibit iron metabolism. These effects are believed to be related to a decrease in lipid peroxidation and iron levels, along with an upregulation of GPX4 expression and a downregulation of ACSL4 expression [27]. Moreover, galangin has been shown to improve learning and memory functions in MCAO/R hamsters by mediating the SLC7A11/GPX4 pathway, reducing lipid peroxide accumulation, and inhibiting ferroptosis [28].
The systemic xc−/GPX4 axis is the key pathway to inhibit ferroptosis. According to the reports, several extractions from TCMs exert their antiferroptosis effects through this axis. Ginsenoside Rb, isolated from Panax ginseng C.A.Mey. or Panax quinquefolium L., was reported to restore system xc activity and inhibit oxidative stress and ferroptosis to exert neuroprotective effects [29]. Moreover, due to the close relationship between ferroptosis and oxidative stress, some researchers also reported that oxidative stress-related signalling pathways including hypoxia-inducible factor (HIF)-1α/ceruloplasmin loop [30], PI3K/Akt/mTOR signalling pathway [31], PI3K-Akt-FoxO signalling [32], and TLR4/p38MAPK pathway [33] may be the target of TCMs to inhibit ferroptosis. In all, the sources and mechanisms of TCM monomers that alleviate cerebral ischemic disease by inhibiting ferroptosis are summarized in Table 1. Additionally, several crude extracts and compound formulas of TCMs that have also been proven to alleviate cerebral ischemic disease by inhibiting ferroptosis are concluded in Table 2.
| Structural classification | Name | Source of TCM | Mechanism | Experimental models | Reference |
|---|---|---|---|---|---|
| Flavonoids | Vitexin | Ficus deltoidea, Spirodela polyrhiza, Vitex negundo | Active Keap1/Nrf2/HO-1 signalling | OGD/R-treated neuron cells, rat MCAO/R model | [16] |
| Quercetin | Ginkgo biloba L., Propolis, Dysosma versipellis (Hance) M. Cheng ex Ying, Bupleurum chinense DC., Eucommia ulmoides Oliver, Epimedium brevicornum Maxim., etc. |
Shuttle labile iron from cell compartments followed by its transfer to transferrin Active/HO-1 signalling pathway |
Rat MCAO/R model | [17] | |
| Baicalein | Scutellaria baicalensis Georgi. | Regulating GPX4/ACSL4/ACSL3 axis, Regulate SIRT6-mediated FOXA2 deacetylation to promote SLC7A11 expression | Mice MCAO/R model, OGD/R-treated HT22 cells | [34, 35] | |
| Icariside II | Epimedium brevicornum Maxim. | Targeting Nrf2 and the OXPHOS/NF-κB/ferroptosis pathway | OGD/R-treated primary astrocytes, mice MCAO/R model | [23] | |
| Calycosin | Astragalus membranaceus (Fisch.) Bge. | Suppress ACSL4-dependent ferroptosis | Rat MCAO/R model, OGD/R-treated PC12 cells | [36] | |
| Puerarin | Pueraria lobata (Willd.) Ohwl, Pueraria thomsonii Benth. | Modulate autophagy and Nrf2 pathway | OGD/R-treated PC12 cells | [37] | |
| Chrysin | Scutellaria indica L., Oroxylum indicum (L.) Vent. | Regulate HIF-1α/CP loop | Rat MCAO/R model, OGD/R-treated PC12 cells | [30] | |
| Farrerol | Rhododendron dauricum L. | Active Nrf2 Pathway | Neonatal rat model of HIE | ||
| Phenolic compounds | Gastrodin | Gastrodia elata Bl. | Active Nrf2/Keap1-GPx4 signalling pathway | BCCAO-induced vascular dementia models | [21] |
| Loureirin C | Dracaena cochinchinensis (Lour.), S.C.Chen, Pelargonium hortorum. | Active Nrf2 pathway | Mouse MCAO/R model, OGD/R-treated primary neurons and SH-SY5Y cells | [22] | |
| Kaempferol | Kaempferia galanga and some zingiberaceae. | Active Nrf2/SLC7A11/GPX4 Axis | OGD/R-treated primary neurons | [38] | |
| Rosmarinic acid | Rosmarinus officinalis, Mentha piperita, Salvia officinalis, etc. | Inhibit TfR1 to attenuate ACSL4/LPCAT3/Lox activation-induced ferroptosis | Wild type and TfR1EC cKO mouse MCAO/R model | [39] | |
| Resveratrol | Polygonum cuspidatum, Veratrum album, Polygonum multiflorum, etc. | Inhibit ferroptosis and reduce degenerative neurons | Rat MCAO/R model, OGD/R-treated PC12 cells | [40] | |
| Caffeic acid | Artemisia capillaries Thunb., Cynara scolymus L., Lonicera japonica Thunb., Daucus carota L., Stachys glabra Bge., Fagopyrum esculentum Moench., Elaeagnus multiflora Thunb., etc. | Nrf2 signalling pathway | Rat MCAO/R model, OGD/R-treated PC12 cells, LPS-treated microglia | [41] | |
| Carvacrol | Mentha, Thymus, etc. | — | Gerbil MCAO/R model | [42] | |
| Saponins | Astragaloside IV | Astragalus membranaceus (Fisch.) Bge. var. mongholicus (Bge.) Hsiao. | Inhibition of P62/Keap1/Nrf2 pathway-mediated ferroptosis | Rat MCAO/R model | [24] |
| Decreased m6 A levels of Acsl4 | OGD/R-treated SH— | [43] | |||
| Glycyrrhizin | Glycyrrhiza uralensis Fisch., Glycyrrhiza glabra L., Glycyrrhiza inflata Batal. | Inhibit HMGB1/GPX4 Pathway | Neonatal rat HIBD model, OGD/R-treated cortical neuron | [44] | |
| Ginsenoside Rb | Panax ginseng C.A.Mey., Panax quinquefolium L. |
Restore System xc activity Activation of NRG1/ErbB4-mediated PI3K/Akt/mTOR signalling pathway |
Neonatal rat hypoxic–ischemic brain damage model, rat MCAO/R model, OGD/R-treated PC12 cells | [29, 31] | |
| Alkaloid | Voacangine | Voacanga africana | Activating the PI3K-Akt-FoxO signalling | OGD/R-treated HT22 cells | [32] |
| Oxysophoridine | Sophora alopecuroides L. | Inhibition of TLR4/p38MAPK-mediated ferroptosis | Rat MCAO/R model, OGD/R-treated HT22 cells | [33] | |
| Volatile oil | β-Caryophyllene | Oleum caryophyll, Artemisia argyi, Eucommia ulmoides | Active NRF2/HO-1 signalling pathway | Rat MCAO/R model | [15] |
| Anthraquinones | Rhein | Polygonum cuspidatum Sieb. et Zucc. | Active NRF2/SLC7A11/GPX4 pathway | Rat MCAO/R model, OGD/R-treated HT22 cells | [20] |
| 15,16-Dihydrotanshinone I | Salvia miltiorrhiza | Active Nrf2 pathway | Rat MCAO/R model, Tert-butyl hydroperoxide-injured PC12 cells | [45] | |
| Terpenoids | 11-keto-β-boswellic acid | Boswellia serrate | — | Rat MCAO/R model | [46] |
| Iridoids | Rehmannioside A | Rehmannia glutinosa Libosch. | Activating PI3K/AKT/Nrf2 and SLC7A11/GPX4 signalling pathway | Rat MCAO/R model, H2O2-treated SH-SY5Y cells | [25] |
| Lactone | Ginkgolide B | Ginkgo biloba L. | Disrupting NCOA4-FTH1 interaction | Rat MCAO/R model, OGD/R-treated PC12 cells | [47] |
| Steroids | Ecdysterone | Cyanotis arachnoidea C.B.Clarke. | Inhibit ferroptosis in neurons through ACSL4 | Rat MCAO/R model, OGD/R-treated PC12 cells | [48] |
- Abbreviations: ACSL, Acyl CoA synthase; BCCAO, bilateral common carotid artery occlusion; FoxO, forkhead box O; GPX4, glutathione peroxidase 4; HIBD, hypoxic–ischemic brain damage; HIF, hypoxia-inducible factor; HO-1, heme oxygenase-1; LPCAT3, lysophosphatidylcholine Acyltransferase 3; LPS, Lipopolysaccharide; MCAO/R, middle cerebral artery occlusion/reperfusion; NCOA4, nuclear receptor coactivator 4; Nrf2, nuclear factor erythroid 2-related factor 2; OGD/R, oxygen–glucose deprivation and reoxygenation; SIRT, silent information regulator; SLC7A11, Solute Carrier Family 7 Member 11; TCM, traditional Chinese medicine.
| Category | Name | Main active ingredients or TCM components | Mechanism | Experiment models | References |
|---|---|---|---|---|---|
| Crude extracts | Neutral polysaccharide from Gastrodia elata | Polysaccharides | Activate NRF2/HO-1 signalling pathway | Mice IS model, OGD/R HT22 cell | [49] |
| Cottonseed oil | Palmitic acid, stearic acid, arachidic acid, oleic acid, linoleic acid, etc. | — | Rat MCAO model | [50] | |
| Paeoniae Radix Rubra extract | Baiyaoside, paeoniflorin, benzoylpaeoniflorin, oleanolic acid, etc. | Active PI3K/Akt signalling pathway | Rat MCAO model, OGD/R HT22 cell | [51] | |
| HJ11 | Si-Miao-Yong-An decoction (honeysuckle flowers, Scrophularia root, Chinese Angelica root, Licorice root). | Inhibit ACSL4 | Myocardial I/R rat model, OGD/R H9c2 cell | [52] | |
| Preparations of TCM | Tongluo decoction | Astragalus membranaceus, Codonopsis pilosula, Atractylodes macrocephala, Angelica sinensis, Chuanxiong, Cinnamomum cassia, Paeonia lactiflora, Polygonum multiflorum, licorice, etc. |
Inhibits endoplasmic reticulum stress activating the Sonic Hedgehog pathway Regulating Nrf2/ARE/SLC7A11 signalling pathway |
Rat MCAO model, OGD/R PC12 cell model Rat transient MCAO models, OGD/R HUVECs cell model |
[53, 54] |
| Tongqiao Huoxue decoction | Paeoniae Radix Rubra, Chuanxiong Rhizoma, Persicae Semen, Carthami Flos, Allii Fistulosi Bulbus, Zingiberis Rhizoma Recens, Jujubae Fructus, etc. | Facilitate ACSL4 ubiquitination degradation | OGD/R-treated PC12 cells, transient MCAO animals | [55] | |
| Xingnaojing injection | Moschus, Curcumae Radix, Borneolum, Gardenia jasminoides Ellis. | — | Rat MCAO model and SH-SY5Y cells | [56] | |
| Extract of Naotaifang | Radix Astragali, Rhizoma Chuanxiong, Bombyx Batryticatus, and Pheretima | Regulate microglial M1/M2 polarization through BMP6/SMADs signalling pathway | Rat MCAO model | [57] | |
| OGD/R BV2 microglia | [58] | ||||
| Danlou tablet | Trichosanthes kirilowii Pericarp, Allium macrostemon Bulb, Pueraria Lobata Root, Chuanxiong Rhizome, Salviae miltiorrhizae Radix et Rhizoma, Paeoniae Radix Rubra, Alismatis Rhizoma, Astragali Radix, Drynariae Rhizoma, Curcumae Radix. | Reduce oxidative stress and COX2 protein levels | Mice transient MCAO model, OGD/R endothelial cells | [59] | |
| Fo-Shou-San | Angelica sinensis, Ligusticum chuanxiong, etc. | Activate NRF2/HO-1 Pathway | Chronic cerebral hypoperfusion mice model | [60] | |
| Danhong injection | Salviae miltiorrhizae Radix et Rhizoma, Carthami Flos. | Upregulate SATB1/SLC7A11/HO-1 Axis | Mice MCAO model | [61] | |
| Angong Niuhuang Wan | Bezoar (Calculus Bovis), Concentrated powder of water buffalo horn, musk, pearl, cinnabar, realgar, coptis root, scutellaria root, gardenia fruit, curcuma root, Borneol. | Activating PPARγ/AKT/GPX4 pathway | Rat MCAO/R model, OGD/R-treated PC12 cells | [62] | |
| Xinglou Chengqi decoction | Trichosanthes cucumeroides, Arisaema erubescens processed with bile juice, Rhei Radix et Rhizoma, sodium sulphate decahydrate | Inhibit SLC7A11/GPX4 Signalling | MCAO/R rat model | [63] | |
| Shenmai injection | Red ginseng, Ophiopogon japonicus | Active Nrf2/GPX4 Signalling pathway | Rat myocardial I/R model | [64] | |
| Zhilong Huoxue Tongyu capsule | Water leech, earthworm, notoginseng, chuanxiong rhizome, common agkistrodon, astragalus root, peach seed, safflower, red peony root, pueraria root, cyathula root | Active PI3K/AKT/Nrf2 axis | Mouse myocardial I/R model | [65] |
- Abbreviations: ACSL, Acyl CoA synthase; BMP6, bone morphogenetic protein 6; COX2, cyclooxygenase-2; GPX4, glutathione peroxidase 4; HO-1, heme oxygenase-1; HUVECs, human umbilical vein endothelial cells; I/R, ischemia reperfusion; IS, ischemic stroke; MCAO, middle cerebral artery occlusion; Nrf2, nuclear factor erythroid 2-related factor 2; OGD/R, oxygen–glucose deprivation and reoxygenation; SLC7A11, Solute Carrier Family 7 Member 11.
5.2 TCMs Alleviate Myocardial Ischemic Disease via Inhibiting Ferroptosis
In clinical practice, angina pectoris, myocardial infarct, and ischemic cardiomyopathy are common myocardial ischemic diseases. These diseases were often investigated using myocardial I/R animal model or OGD/R cell model. Myocardial I/R injury occurs when blood supply returns to the tissue after a period of ischemia or lack of oxygen. This jeopardizes the heart muscle, commonly seen in conditions such as myocardial infarction, where blockage of coronary arteries leads to insufficient blood flow, subsequently leading to heart tissue damage. The mortality rate associated with myocardial I/R injury remains significant, contributing substantially to cardiovascular morbidity and mortality worldwide. Current therapeutic approaches to mitigate I/R injury include mechanical reperfusion strategies, such as percutaneous coronary intervention and coronary artery bypass grafting, pharmacological treatments involving antiplatelet and antithrombotic agents, and emerging therapies like ischemic preconditioning and postconditioning.
Several studies have shown that various Chinese herbal formulations exhibit anti-inflammatory and antioxidant properties, which may help reduce oxidative stress, lipid peroxidation, and ferroptosis in ischemic hearts. TCM and its compound formulations offer an integrative approach to alleviating myocardial I/R injury, leveraging centuries of clinical experience and modern scientific validation. Compound preparations or extractions such as Shenmai Injection [64], Zhilong Huoxue Tongyu capsule [65], and HJ11 [52] that can inhibit ferroptosis and alleviate myocardial ischemia are concluded in Table 2.
Recent studies that reported notable candidates of TCMs including ginsenosides from Panax ginseng, curcumin from Curcuma longa L., and Puerarin from Pueraria lobata (Willd.) Ohwi can act as a potent ferroptosis inhibitor and alleviate myocardial ischemia diseases in mice or rat myocardial I/R model or OGD/R-treated H9c2 cells. Structurally, flavonoids, phenolic acids, and terpenoid structures are the most investigated Chinese herbal monomer components, and the mechanisms of these compounds activate Nrf2 and GPX4 signalling pathways, which are concluded in Table 3. Moreover, for the reason that elevated lipid peroxidation and ROS are important features leading to ferroptosis, in these studies, extensive attention was paid to the level of lipid peroxidation products and the expression levels of ferroptosis-related proteins, such as GPX4, ACSL4, Ferritin, and transferrin receptor 1 (TfR1). However, the morphological changes of cells such as decreased mitochondrial size, increased membrane density, decreased or disappeared cristae, and broken outer membrane have not received widespread attention. These agents target various pathways involved in iron metabolism, oxidative stress, and lipid peroxidation, making them promising candidates for therapeutic interventions in ischemic diseases. By alleviating oxidative stress and preventing ferroptosis, these agents may not only mitigate the symptoms of angina pectoris but also improve overall cardiovascular health.
| Structural classification | Name | Source of traditional Chinese medicine | Mechanism | Experimental models | Reference |
|---|---|---|---|---|---|
| Flavonoids | Anthocyanin-3-glucoside | Wildly distributed in purple or red vegetables and fruits | Inhibit oxidative stress and autophagy, upregulate FTH-1 and GPX4 inhibiting ferroptosis | Rat myocardial I/R model, OGD/R-induced H9c2 cells | [66] |
| Naringenin | Citrus grandis (L.) Osbeck, Citrus paradisi, etc. | Active Nrf2/System xc-/GPX4 axis | Rat myocardial I/R model, OGD/R-induced H9c2 cells | [65] | |
| Xanthohumol | Humulus lupulus L. | — | OGD/R-induced H9c2 cells | [67] | |
| Luteolin | Reseda odorata L., Arachis hypogaea L., Perilla frutescens (L.) Britt., etc. | Inhibit ACSL4 and Nrf2 | OGD/R-induced H9c2 cells | [68] | |
| Baicalein | Scutellaria baicalensis Georgi, Scutellaria altissima L., Scutellaria scrodiifolia Fisch., etc. | Inhibit ACSL4 and Nrf2 | OGD/R-induced H9c2 cells | [68] | |
| Puerarin | Pueraria lobata (Willd.) Ohwi, Pueraria thunbergiana Benth. | — | Mice myocardial I/R model, OGD/R-induced H9c2 cells | [37, 69] | |
| Icariin | Epimedium brevicornum Maxim., Epimedium sagittatum (Sieb. et Zucc.) Maxim., Epimedium pubescens Maxim., etc. | Inhibit IRE1/JNK-induced ferroptosis | OGD/R-induced H9c2 cells | [70] | |
| Nobiletin | Citrus reticulata, Citrus unshiu, etc. | Inhibit ACSL4 and NCOA4-related ferroptosis | T2DM Rat myocardial I/R model, HFHG OGD/R-induced H9c2 cells | [71] | |
| Galangin | Alpinia officinarum Hance | Activate Nrf2/GPX4 Signalling Pathway | Mice myocardial I/R model OGD/R-induced neonatal rat cardiomyocytes | [72, 73] | |
| Hydroxysafflor Yellow A | Carthamus tinctorius L. | Activation of the HIF-1α/SLC7A11/GPX4 Signalling Pathway | Mice myocardial I/R model, OGD/R-induced H9c2 cells | [74] | |
| Licochalcone A | Glycyrrhiza uralensis Fisch, Glycyrrhiza glabra L., Glycyrrhiza inflata Batal. | — | Rat myocardial I/R model | [75] | |
| Isoliquiritigenin | Glycyrrhiza uralensis Fisch. | Regulate the Nrf2/HO-1/SLC7A11/GPX4 axis | Mice myocardial I/R model, OGD/R-induced neonatal mice cardiomyocytes | [76] | |
| Polyphenols | Curcumin | Curcuma longa L., Curcuma aromatica Salisb., C. zedoaria (Berg.) Rosc., Acorus calamus L., Acorus calamus L., etc. | Enhance HES1 protein expression | Mouse myocardial I/R model, myocardial I/R model | [77] |
| Resveratrol | Vitis vinifera L., Polygonum cuspidatum Sieb. et Zucc., etc. | Reduce oxidative stress and attenuate ferroptosis | OGD/R-induced H9c2 cells | [78] | |
| VDAC1/GPX4 pathway | Myocardial I/R model, OGD/R-induced H9c2 cells | [79] | |||
| Piceatannol | Vitis vinifera, Vaccinium spp., Passiflora edulis | Regulate Nrf2 signalling-mediated iron metabolism | Mouse myocardial I/R model | [80] | |
| Terpenoids | Gossypol Acetic Acid | Gossypium spp. | Reduce cell death and lipid peroxidation of myocardial cells | Rat myocardial I/R model, OGD/R-induced H9c2 cells, OGD/R-induced neonatal rat cardiomyocyte cells | [81] |
| Pachymic acid | Poria cocos, Ganoderma lucidum | Activate AMPK pathway | OGD/R-treated HL-1 cardiomyocytes | [82] | |
| Ginsenoside Re | Panax ginseng C.A.Mey. | Inhibit miR-144-3p/SLC7A11 pathway | Rat myocardial I/R model | [83] | |
| Glycoside | Kinsenoside | Anoectochilus roxburghii | Activate Akt/Nrf2/HO-1 pathway | Mice myocardial I/R model, OGD/R-induced H9c2 cells | [84] |
| Glycyrrhizin | Glycyrrhiza uralensis Fisch., Glycyrrhiza glabra L., etc | Inhibit HMGB1-TLR4-GPX4 pathway | Rat myocardial I/R model, OGD/R-induced H9c2 cells | [85] | |
| Salidroside | Rhodiola crenulata, Rhodiola rosea | Mitochondrial superoxide-dependent AMPKα2 activation | Rat myocardial I/R model, OGD/R-induced H9c2 cells | [86] | |
| Saponins | Hederagenin | Hedera helix, Clematis chinensis Osbeck. | Attenuate ALOX5-mediated ferroptosis |
Rat myocardial I/R model OGD/R-induced H9c2 cells |
[87] |
| Notoginsenoside R1 | Panax notoginseng (Burk.) F.H. Chen. | Facilitated Nrf2 nuclear translocation | High-altitude myocardial injury | [88] | |
| Ginsenoside Rg3 | Panax ginseng, Panax quinquedolius | Active keap1/Nrf2/GPX4 signalling pathway | Mouse myocardial I/R model, OGD/R-induced H9c2 cells | [89] | |
| Phenolic acid | Ferulic Acid | Angelica sinensis, Ligustincum chuanxiong, Cimicifuga foetida, Ziziphus jujuba var. spinosa, Equisetum hiemale | Upregulating AMPKα2 expression-mediated ferroptosis depression | Rat myocardial I/R model | [90] |
| Salvianolic Acid B | Salviae miltiorrhizae Radix et Rhizoma | Decrease the ubiquitin-proteasome degradation of GPX4 and the ROS-JNK/MAPK Pathways | Rat myocardial I/R model, OGD/R-induced H9c2 cells | [91] | |
| Tanshinone IIA | Salvia miltiorrhiza | Increase VDAC1 protein expression | OGD/R-induced H9c2 cells | [92] | |
| Polysaccharide | Polysaccharides from Chuanminshen violaceum | Chuanminshen violaceum | — | OGD/R-induced H9c2 cells, Mice myocardial I/R model | [93] |
| Alkaloid | Berberine | Coptis chinensis Franch., Phellodendron chinense Schneid., Nandina domestica, Berberis virginiana, Thalictrum fortune, etc. | — | OGD/R-induced neonatal rat cardiomyocyte cells, OGD/R-induced H9c2 cells | [94] |
- Abbreviations: ACSL, Acyl CoA synthase; ALOX5, arachidonate 5-lipoxygenase; AMPK, Adenosine 5′-monophosphate-activated protein kinase; FTH-1, ferritin heavy chain; GPX4, glutathione peroxidase 4; HES1, hes family bHLH transcription factor 1; HIF, hypoxia-inducible factor; HMGB1, high mobility group box-1 protein; I/R, ischemia/reperfusion; NCOA4, nuclear receptor coactivator 4; Nrf2, nuclear factor erythroid 2-related factor 2; OGD/R, oxygen–glucose deprivation and reoxygenation; VDAC1, Voltage Dependent Anion Channel 1.
5.3 TCMs Alleviate Peripheral Arterial Ischemia Diseases via Inhibiting Ferroptosis
Peripheral artery ischemia disease is a common circulatory condition characterized by narrowing or blockage of peripheral arteries, often leading to critical complications such as lower limb ischemia and I/R injury. These conditions can result from various factors, including diabetes, atherosclerosis, and other vascular diseases. Recent studies have highlighted the role of ferroptosis in the pathophysiology of peripheral artery ischemic injuries. Syringic acid, a phenolic compound found in various plants, has been shown to suppress ferroptosis in skeletal muscle cells, alleviating lower limb I/R injuries in animal models by modulating the high mobility group box-1 (HMGB1) signalling pathway [95]. Similarly, salidroside, a bioactive glycoside derived from Rhodiola rosea, has demonstrated the ability to promote therapeutic angiogenesis in diabetic hindlimb ischemia by inhibiting ferroptosis [96]. Rutin, a widely studied flavonoid present in many fruits and vegetables, has garnered interest due to its potent antioxidant properties. Research indicates that rutin can prevent pulmonary arterial hypertension by mitigating ferroptosis in both animal models and pulmonary artery smooth muscle cells exposed to hypoxic conditions [97]. These findings offer new avenues for therapeutic intervention.
5.4 TCMs Alleviate Renal Ischemia Diseases via Inhibiting Ferroptosis
Renal ischemic diseases can arise from multiple causes, including renal artery stenosis, acute kidney injury (AKI), and systemic issues such as shock or severe dehydration. The consequences of renal ischemia are severe, with potential progression to chronic kidney disease (CKD), renal fibrosis, and even end-stage renal disease (ESRD). In NRK-52E cells, HK-2 cells as well as I/R mice, quercetin, a natural flavonoid, can act as a ferroptosis inhibitor via activating activation transcription factor 3 (ATF3) [98]. Paeoniflorin, a bioactive monomer extracted from Paeonia lactiflora, has also shown promise in alleviating I/R-induced AKI by inhibiting ferroptosis mediated by SLC7A11 [99]. Furthermore, curcumin, derived from the spice turmeric (Curcuma longa), mitigates ferroptosis in hepatic, pancreatic, and cardiac tissues in I/R models by enhancing ACSL/GPX4 signalling pathways [100]. Jiang et al. [101] proved a similar effect of pachymic acid. Additionally, puerarin, originating from Pueraria lobata, has been reported to reduce oxidative stress and ferroptosis associated with renal fibrosis due to ischemia [102]. Salidroside, a phenylpropanoid glycoside found in Rhodiola rosea, inhibits ferroptosis induced by renal I/R injury through the PI3K/AKT signalling pathway [103]. Furthermore, vitexin, extracted from Crataegus spp., has been demonstrated to alleviate CKD by inhibiting ferroptosis in renal tubular epithelial cells via Nrf2 activation [104]. Loureirin C, a compound from Resina draconis, enhances mitochondrial function by promoting the nuclear translocation of Nrf2, thereby reducing oxidative damage from renal I/R injury [105]. Silibinin, derived from milk thistle (Silybum marianum), has been shown to mitigate ferroptosis in acute kidney injury by targeting ferritin heavy chain (FTH1) [106]. These findings collectively underscore the potential of TCMs and their active components in addressing ferroptosis and promoting renal protection in the context of ischemic-related diseases.
5.5 TCMs Alleviate Intestinal Ischemia Diseases via Inhibiting Ferroptosis
Intestinal ischemic diseases occur primarily during episodes of reduced blood flow to the intestines, often triggered by conditions such as intestinal obstruction, mesenteric artery embolism, or thrombus formation. Commonly used models include the intestinal I/R model, which replicates the hemodynamic changes seen in human patients and can allow researchers to assess both acute and chronic phases of ischemia. Recent studies have focused on the active component found in chilli peppers, capsite, which can protect mice from intestinal ischemia by inhibiting ferroptosis, thereby decreasing oxidative stress and promoting cell survival via activating transient receptor potential vanilloid-1 and increasing GPX4 protein expression [107]. Isoliquiritin apioside, a critical ingredient of Glycyrrhizae radix et rhizoma relieves intestinal I/R-induced acute lung injury by blocking HIF-1α-mediated ferroptosis [108]. A well-known antioxidant, resveratrol, can be isolated from Veratrum album, Polygonum cuspidatum, and many TCMs. It has been reported that resveratrol reduces ROS-induced ferroptosis, the underlying mechanism includes activating SIRT3 and compensating the GSH/GPX4 pathway [109]. Similar effects also have been found by naringenin via targeting YAP/STAT3 signalling axis [110]. Apigenin-7-O-Glucoside (AGL) is a sugar derivative of apigenin, belonging to the flavone compound with poor water solubility. DTPA-N10-10 and mPEG-TK-DA self-assembled to encapsulate AGL through hydrophilic and hydrophobic interactions forming multisite ROS scavenging nanoparticles termed PDN@AGL. PDN@AGL was reported to alleviate intestinal I/R by ATF3/SLC7A11-mediated ferroptosis [111].
5.6 TCMs Alleviate Ischemic Liver Disease via Inhibiting Ferroptosis
Hepatic ischemic diseases result from inadequate blood flow to the liver, leading to significant hepatocyte injury and dysfunction. These conditions frequently occur during scenarios such as hepatic artery embolism, hepatic surgery, and liver transplantation, where blood supply may be temporarily interrupted, leading to high morbidity and mortality rates. Tanshinone functions as a coenzyme that confers the gain of function of NQO1 to suppress ferroptosis, followed by reducing ferroptotic cell death in hepatocytes, thus alleviating acute liver injury [112]. Through enhancing Nrf2/HO-1/GPX4 activity, fucoidan mitigates oxidative stress and inhibits ferroptosis in liver tissues following ischemic events, making them valuable in managing I/R injury in liver transplantation [113]. Normally, due to the high risk of I/R injury, severe hepatic steatosis in the donor liver prohibits transplantation. Oleanolic acid has been reported can alleviate I/R injury and inhibit ferroptosis in severe steatosis liver. Network pharmacology and molecular docking implicated Keap1/NRF2 pathway as a potential target [114].
5.7 TCMs Alleviate Other Ischemic Diseases via Inhibiting Ferroptosis
Recent studies have highlighted some active ingredients and preparations of TCMs, in managing conditions like ocular ischemia and lung I/R injuries. Wolfberry, celebrated for its health benefits, contains polysaccharides known for their antioxidant properties. A recent study reported that Lycium barbarum polysaccharide-derived nanoparticles preserve visual function in response to retinal vascular occlusion by activating the Nrf2 pathway, thus protecting retinal ganglion cells from ferroptosis [115]. Rhodiola rosea has been shown to enhance cellular resilience in hypoxic conditions. Salidroside is a potent active compound of Rhodiola rosea. In models of ischemic brain or eye scenarios, salidroside facilitates metabolic recovery and diminishes iron overload, thus preventing ferroptosis. Moreover, these effects may be due to inhibiting JAK2/STAT3 pathway activation in lung I/R [116]. Ferroptosis has also been proven to play important roles in endometrial injury [117], bone ischemia, including avascular necrosis of the femoral head [118-120]; however, the effects of TCMs remain for further investigation.
6 Research Gaps and Limitations
The current research on the inhibitory effects of TCM on ferroptosis mainly focuses on the modulation of classical signalling pathways rather than the direct interactions between TCM monomers and specific protein targets. Due to a lack of clinical trials, pharmacokinetic parameters and individual differences are difficult to predict and evaluate, which may increase the risk and uncertainty of treatment. Therefore, more research on the efficacy of natural products, such as the discovery of new targets, optimization of drug combinations, in-depth exploration of the mechanism, and more clinical trials, are needed.
The complexity of TCM formulations, which often consist of multiple compounds, poses a challenge in identifying specific active ingredients responsible for ferroptosis inhibition. The interactions among these compounds may result in synergistic effects that are difficult to quantify. Furthermore, the mechanisms underlying TCM's effects on ferroptosis are still not fully elucidated, hindering the establishment of clear therapeutic pathways. Second, many existing studies rely on preclinical models, which may not adequately replicate human pathophysiology. There is a pressing need for clinical trials to confirm the efficacy and safety of TCM interventions in patients with ischemic diseases. Additionally, substantial variability in individual responses to TCM can complicate the interpretation of results. To address these limitations, advanced technologies such as single-cell RNA sequencing (scRNA-seq) and metabolomics offer promising avenues for future research. ScRNA-seq allows for high-resolution profiling of cellular responses at the individual cell level, enabling researchers to identify specific cell populations that may be particularly vulnerable to ferroptosis. This technology can help delineate how TCM affects various cell types in the context of ischemia, providing insights into the cellular mechanisms of action and potential biomarkers for patient stratification. Moreover, metabolomics, which focuses on the comprehensive study of metabolites within cells, tissues, or biofluids, can be instrumental in elucidating the metabolic alterations associated with TCM treatment. By quantifying changes in metabolic profiles, researchers can gain insights into how TCM modulates iron homeostasis and lipid metabolism, the two critical components of ferroptosis. This approach could help identify novel therapeutic targets and metabolic pathways influenced by TCM.
Moreover, TCMs extract may have poor water solubility, leading to a decrease in bioavailability. For drugs with good biological activity, the preparation of nanoparticles, liposomes, or other carriers can be considered to enhance the solubility and absorption rate of the drug. In addition, the supervision and standardization of the quality of TCMs should also be given attention, for example, establishing a quality traceability system for TCMs and improving regulations to ensure safety and effectiveness in clinical applications.
7 Outlook and Conclusions
Ischemic-related diseases remain a significant global health burden, with high morbidity and mortality rates associated with conditions such as ischemic heart disease, stroke, and peripheral artery disease. Despite advancements in treatment modalities, the clinical efficacy of existing therapies is often limited by various factors, including adverse effects, drug resistance, and a heterogeneous patient population. The pathophysiological complexities of ischemic-related diseases necessitate a deeper understanding of their underlying mechanisms to unveil novel therapeutic targets.
Recent studies have indicated that inhibiting ferroptosis is a critical target for improving ischemic-related diseases. TCM is a treasure of Chinese traditional culture, usually characterized by multitarget and high safety. In this review, we primarily summarized TCM monomers, extractions, and compound formulations that can alleviate ischemic diseases by inhibiting ferroptosis. Many TCM monomers and compound formulations have been shown to improve ischemic conditions in a manner associated with ferroptosis, with a significant focus on activating the Nrf2 and GPX4 signalling pathways. Additionally, some research has shown that classical signalling pathways related to oxidative stress also play a role in the regulation of ferroptosis. This may be due to the fact that uncontrolled lipid peroxidation can also be activated by oxidative stress. Thus, future research should also explore the therapeutic potential of combining ferroptosis inhibitors with other approaches such as antioxidant therapy, anti-inflammatory agents, and endothelial function modulators. Our review indicates that polyphenolic and flavonoid compounds have been relatively well studied in their ability to inhibit ferroptosis and improve ischemic-related diseases. Subsequent research exploring structure–activity relationships based on drug targets would be highly meaningful and could provide further insights into their mechanisms. Furthermore, high-throughput screening of TCM compounds may yield novel agents that can effectively modulate ferroptosis and thereby confer protection against ischemic injury.
In conclusion, previous literature reviews have examined the role of TCMs in the process of cerebral ischemia, with a focus on iron death and its interference with other signalling pathways [121]. Our article focuses more on drug mechanisms and targets, which has positive significance for the development of new drug targets and drugs. Understanding the nuances of ferroptosis in the context of ischemic diseases opens new therapeutic avenues that could significantly enhance patient outcomes. The application of TCMs in this domain holds promise, but a concerted effort is required to validate their efficacy and elucidate the underlying mechanisms.
Author Contributions
Yukun Zhang: conceptualization, writing – original draft, writing – review and editing, project administration. Yang Yao: conceptualization, writing – original draft, writing – review and editing. Baoxue Yang: conceptualization, writing – review and editing, supervision. All authors: writing the first draft and revising. Figure 1 was created and designed by Yang Yao and Yukun Zhang. Figure 2 was created and designed by Yang Yao and Yukun Zhang. Table 1 was created by Yukun Zhang. Table 2 was created by Yukun Zhang. Table 3 was created by Yukun Zhang. All authors approved the final manuscript for submission.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China (82304493), the Wanzhou District Social Welfare Research Project (202206151527122088), the Science and Technology Research Project of Chongqing Education Commission (KJZD-M202302701), the Key Laboratory Special Project of Chongqing Three Gorges Medical College (Sys20210002), and the Proof of Concept Project of Medical Innovation Center (Taizhou) of Peking University (Grant CXYZ-2024-101).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analyzed during the current study.