Meta-analysis: Impact of intragastric balloon therapy on NAFLD-related parameters in patients with obesity
As part of AP&T's peer-review process, a technical check of this meta-analysis was performed by Dr Y Yuan. The Handling Editor for this article was Professor Rohit Loomba, and it was accepted for publication after full peer-review.
Summary
Background
Non-alcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease affecting approximately 25% of adults in the western world. Intragastric balloon (IGB) is an endoscopic bariatric therapy -a therapeutic endoscopic tool that has shown promise in inducing weight loss. Its role in the treatment of NAFLD is yet to be established.
Aim
To evaluate the effect of IGB as a treatment option in NAFLD.
Methods
We searched MEDLINE (PubMed) and EMBASE from inception to September 2022. We included studies evaluating the impact of IGB on obesity with the assessment of one or more liver-related outcomes and studies primarily evaluating the impact of IGB on NAFLD. We included comparative and non-comparative studies; primary outcomes were liver-related NAFLD surrogates.
Results
We included 19 studies with 911 patients. IGB demonstrated an effect on NAFLD parameters including NAFLD activity score (NAS): mean difference (MD): −3.0 [95% CI: −2.41 to −3.59], ALT: MD: −10.40 U/L [95% CI: −7.31 to −13.49], liver volume: MD -397.9 [95% CI: −212.78 to 1008.58] and liver steatosis: MD: −37.76 dB/m [95% CI: −21.59 to −53.92]. There were significant reductions in non-liver-related outcomes of body weight, BMI, glycated haemoglobin and HOMA-IR.
Conclusion
Intragastric balloons may play an important role in addressing the treatment gap in NAFLD management.
1 INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is currently the most common cause of chronic liver disease worldwide.1-3 It is a growing cause of end-stage liver disease and is increasingly being associated with hepatocellular cancer.4 NAFLD is intimately linked to other elements of the metabolic syndrome including hypertension, dyslipidaemia and diabetes mellitus. Factors that lead to increased circulating fatty acids and subsequent deposition in the liver parenchyma, for example insulin resistance, have been postulated to be the main driving mechanisms for the development of NAFLD.5
Non-alcoholic fatty liver disease is a disease spectrum that ranges from non-alcoholic fatty liver characterised by simple steatosis to non-alcoholic steatohepatitis (NASH) characterised by steatosis with associated inflammation with or without fibrosis, which may progress to cirrhosis.2, 6, 7 NASH, the more aggressive form, is associated with histologic characteristic features such as hepatocyte injury (ballooning degeneration) and hepatic inflammation with or without fibrosis, with increased risk of progression to cirrhosis, HCC and End-stage liver failure.8-10
Patients with NAFLD may have mild to moderate elevations of transaminases (alanine aminotransferase & Aspartate aminotransferase); however, some could have normal transaminases. The scale of transaminases elevation does not predict the level of hepatic inflammation or fibrosis.11-13
Weight loss is the mainstay of NAFLD management; a focus on intense and sustained weight loss has been proven to be effective for treating NAFLD.14, 15 A sustained weight loss of ≥7%–10% of body weight (BW) is recommended to reverse the process of steatosis, inflammation and fibrosis.16-19 Unfortunately, compliance is a major limiting factor, and only approximately 10%–20% of patients achieve this target.17, 18, 20 To date, neither lifestyle modification (LM) nor NAFLD-specific medications have been shown to be reliable or effective in the treatment of NAFLD. There are no approved medications currently licenced for the management of NAFLD; several drugs have been developed to prevent or slow the progression of hepatic steatosis to inflammation and fibrosis with unsatisfactory results.14, 21, 22 Although bariatric surgery has shown to be highly effective for long-term weight loss and reversal of both diabetes mellitus and NAFLD,23, 24 its use is limited by strict eligibility criteria, cost and access to expert centres.25 As a result of these inadequacies, the majority of NAFLD patients are left untreated.
Endoscopic bariatric therapies (EBTs) have emerged as potentially safe and effective treatment options for obesity and its associated comorbid conditions.26, 27 EBTs were developed to avoid the invasive nature of bariatric surgery, while at the same time reproducing its physiological alterations and therapeutic effects.28 EBTs consist of gastric and small bowel devices/techniques; Gastric EBTs include temporary space occupying devices such as Intragastric balloons (IGBs), while a Transpyloric shuttle functions to close off the pylorus intermittently, leading to both delayed gastric emptying and subsequent prolonged satiety. Gastric remodelling techniques such as Endoscopic Sleeve Gastroplasty (ESG) reduce the gastric volume through an intragastric suturing device (Overstitch by Apollo Endosurgery, Endomina by Endo tools therapeutics). Another remodelling technique called POSE (Primary obesity surgery endoluminal), uses an incisionless plication device, to create full-thickness suture plications in the gastric fundus and body. In a recently published randomised controlled trial on the effect of POSE 2.0 (a modification of the original POSE) on NAFLD, there was a significant reduction in hepatic steatosis, liver enzymes, AST-to-platelet ratio Index and %Total Body Weight Loss (TBWL) at 12 months in patients who underwent POSE.29 Small bowel EBTs prevent duodenal absorption of luminal contents, either through EndoBarrier, which is a duodenal-jejunal bypass liner (DJBL) or mucosal hydrothermal ablation using the Revita system for duodenal mucosal resurfacing (DMR). Several studies have shown that EBTs are efficacious in inducing weight loss, ranging from 10% - 30% TBWL, with majority of patients achieving at least 10% TBWL. As a result, EBTs have the potential to play an important role in the management of NAFLD; several recent studies have demonstrated the potential of ESG, DJBL and DMR.30-33 Furthermore, a number of meta-analyses have highlighted the efficacy of EBTs in the management of NAFLD, leading to improvement of key NAFLD parameters—liver fibrosis, liver steatosis and liver Enzymes.34-36 While these studies examined the effects of all EBTs, the main focus of this meta-analysis was Intragastric Balloons (IGBs) which are the most popular form of EBT.
IGBs are temporary space occupying devices that induce weight loss through early intrameal satiety and delayed gastric emptying, leading to reduced caloric intake; In addition, they have a good safety profile.37 A multicentre, prospective, randomised trial including 255 obese participants showed that subjects randomised to the IGB group + Lifestyle intervention, had a higher total body weight loss at 6 months in comparison to subjects randomised to lifestyle intervention alone. Additionally, the difference in weight loss between the two randomised groups, was maintained at 12 months.38 The silicone based Orbera365, formerly known as Bioenterics Intragastric Balloon (Allergan), is the most popular IGB. It is indicated for adults with obesity with a body mass index (BMI) ≥30 and ≤40 kg/m2. It is placed endoscopically in the corpus and filled with 450–700 mL of saline.
The aim of this meta-analysis was to evaluate the impact of Intragastric Balloons on NAFLD outcomes—NAFLD Activity score (NAS), liver enzymes, liver volume, liver steatosis, liver fibrosis and non-liver-related outcomes—glycated haemoglobin (HBa1c), body mass index (BMI), total body weight loss (TBWL), insulin resistance via Homeostatic Model Assessment for Insulin Resistance (HOMA-IR).
Of note, majority of the studies included focused on the use of IGB as a tool in the treatment of obesity, liver-related outcomes were evaluated as secondary endpoints. The most commonly evaluated outcome were liver enzymes. In the overall cohort of included patients, approximately 50% had a presumed diagnosis of NAFLD/NASH, with no formal diagnosis described. This presumption was based mostly on elevated liver enzymes.
2 METHODS
2.1 Data sources and search strategy
The systematic review was conducted in line with The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidance.39 Two independent reviewers (OA, TM) interrogated the two online databases, MEDLINE(PubMed) and EMBASE. Additional articles were obtained mainly through citation referencing, by thoroughly scrutinising the reference lists of selected articles and other articles of interest. An extensive strategy was used to search for articles that relate to Intragastric balloon and its effect in the management of patients with non-alcoholic fatty liver disease. The search strategy was done using the following keywords—'Intragastric Balloon’, ‘Intragastric Balloon’, ‘Gastric Balloon’, ‘Non-Alcoholic fatty liver disease’, ‘Non-alcoholic fatty liver disease’, ‘Fatty liver’, ‘Hepatic steatosis’ and ‘Obesity’. The methods were registered a priori on PROSPERO CRD42022374374, and the full search strategy can be found in Data S1.
2.2 Eligibility criteria and data abstraction/extraction
We included randomised clinical trials (RCTs) and observational studies that (1) evaluated the effect of Intragastric balloons on obese patients, reporting at least one liver-related outcome as secondary endpoint and (2) studies primarily evaluating the impact of Intragastric balloons on NAFLD outcomes. We included both single-arm studies (Effect of Intragastric Balloon on NAFLD pre- and post-procedure) and double-arm studies (Intragastric Balloon versus Lifestyle modification or medical therapy or sham procedure). Only published studies were included; conference abstracts, case reports and series, expert opinions, editorials and review articles were excluded.
Independent reviewers (OA, TM) screened articles at full text for eligibility or limited screening to title and abstract review if the articles clearly did not meet eligibility criteria. The data were extracted from studies using a purposely designed template.
2.3 Outcomes
The primary outcomes were (1) NAFLD activity score (NAS)—sum of individual NAFLD histologic scores (steatosis + lobular inflammation + ballooning) and (2) liver enzymes—alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT).
The secondary outcomes were (1) liver volume assessed radiologically (2) liver steatosis assessed either histologically (steatosis score) or radiologically—controlled attenuation parameter via fibroscan, hepatic fat fraction from MRI (3) liver fibrosis assessed either histologically (fibrosis score), serologically (NAFLD fibrosis score or APRI) or liver stiffness measurement (LSM) via Fibroscan (4) weight (5) BMI (6) HBA1c and (7) Insulin resistance assessed via HOMA-IR.
Fourteen of the included studies compared pre-treatment outcomes to post-treatment outcomes, while the remaining 5 compared IGB treatment outcomes to that of controls. NAFLD Activity score (NAS) was included as a primary outcome (even though only 2 studies evaluated it) in this meta-analysis, as it is widely considered a key parameter in studies involving NAFLD patients.
2.4 Risk of bias assessment
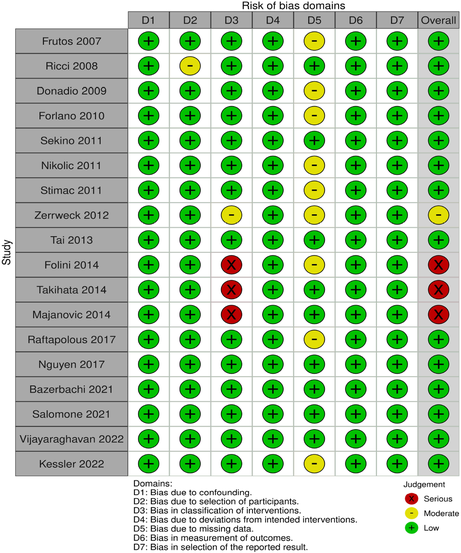
Two independent reviewers (OA, TM) assessed each study for risk of bias. According to the Cochrane Risk of Bias in Non-Randomised studies of intervention tool (ROBINS-I),40 Folini et al,41 Takihata et al42 and Majanovic et al43 showed an overall serious risk of bias due to bias in classification of interventions(Patients were allowed to choose their intervention arm) and bias due to missing data (Patients dropped out before study completion). Furthermore, Zerrweck et al44 showed a moderate risk of bias due to moderate biases in the classification of interventions and missing data. The remaining 15 included studies all showed an overall low risk of bias.
The Cochrane Risk of Bias in randomised studies tool (ROB-2)45 expressed an overall low risk of bias in the only included randomised controlled trial—Lee7 (Tables 1 and 2).
|

|
2.5 Data synthesis and statistical analysis
The data were expressed as mean ± SD and effect estimates as mean difference (MD). Meta-analyses were undertaken using a random-effects model. All continuous outcomes were analysed using the mean difference (MD) and associated 95% confidence intervals (CIs). All statistical tests were 2-sided with a p < 0.05 deemed statistically significant. Statistical heterogeneity was investigated using the I2 test: I2 < 25% denoted ‘low’ heterogeneity; I2 = 25%–50% denoted ‘moderate’ heterogeneity; I2 > 50% denoted ‘high’ heterogeneity. Funnel plots were also included to assess publication bias. All analysis was conducted using the RevMan software (Review Manager Software version 5.4-Cochrane Collaboration Copyright© 2020). Statistical Analysis was overseen by FB, who is a senior lecturer in Biostatistics and Research Methods at the Royal College of Surgeons Ireland (RCSI) Data Science Centre.
3 RESULTS
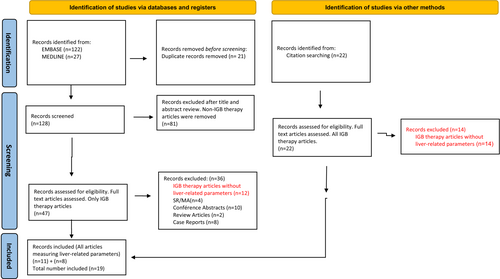
Our search strategy produced a total of 149 articles (122 from EMBASE, 27 from MEDLINE-PubMed), 128 were screened after removal of duplicates. At title/abstract screening, 81 articles mainly focusing on other EBTs, Bariatric surgery and non-invasive therapies were found ineligible and excluded. Forty-seven articles that focused on IGBs underwent full-text retrieval and review; unpublished studies, review articles, meta-analyses, case reports and conference abstracts were excluded. Furthermore, studies on IGBs without liver-related outcomes were excluded. Following full-text review, 11 articles fulfilled the criteria for inclusion in this review. An additional 22 articles were obtained via manual search especially through citation referencing. Eight of these articles fulfilled the inclusion criteria for our review following full-text review. Finally, a total of 19 articles were included in this review as outlined in Figure 1. All 19 articles evaluated liver-related outcomes.
3.1 Study characteristics
A total of 911 participants were enrolled in the included studies. Most of the included studies, were based on the use of Orbera IGB. Of the 19 studies included in this review, only 1 was a randomised controlled trial7—which compared IGB to a sham, 4 studies were comparative observational studies—comparing IGB to cognitive behavioural therapy43 or lifestyle modification.41, 42 Zerrweck et al44 compared gastric bypass with pre-operative IGB treatment to gastric bypass alone. The remaining 14 studies were non-comparative observational studies,46-59 as shown in Tables 3 and 4.
| Study | Study design | N (no) | Age | Study population | Type of IGB | F/up (months) | Liver-related outcomes | NAFLD diagnostic modality | Mean %TBWL or %EWL |
|---|---|---|---|---|---|---|---|---|---|
| Frutos 200746 |
Non-comparative observational Pre-operative IGB therapy prior to gastric bypass |
31 | 40 ± 11 | Obesity | BIB/Orbera | 6 | CT measurement of liver volume; reduction of mean liver volume from 2938.53 ± 853.1 cm3, to 1918.2 ± 499.8 cm3 after 6 months | CT scan | % EWL: 22.14% ± 7.39 in 29/31 |
| Ricci 200847 | Non-comparative observational, Retrospective | 103 (93 eligible) | 41.3 ± 11 | Obesity | BIB/Orbera | 6 | ALT, GGT | NR | Reduction of BMI of ≥10% in 59 patients |
| Donadio 200948 | Non-comparative observational | 40 | 37 ± 11 | Obesity | BIB/Orbera | 6 | AST, ALT, GGT | NR |
13.2 ± 6.5% weight loss observed at BIB removal + reduction of BMI (13.2%) 72.5% achieved weight reduction of at least 10% |
| Forlano 201049 | Non-comparative observational | 130 (120 completed) | 38.6 | Obesity | BIB/Orbera | 6 |
ALT, GGT Liver steatosis improved from 52% to >4% in the 91 responders |
Ultrasound | 57% achieved 10% TBWL, 91 responders with reduced BMI of ≥3.5 kg/m2 |
| Sekino 201150 | Non-comparative observational | 8 | 39 ± 11 | Obesity | BIB/Orbera | 6 | ↓ AST, ALT, GGT; reduction in median liver volume from 1873.3 to 1751.6 after 6 months | CT scan | % EWL: 14.85 in 8/8 |
| Nikolic 201151 | Non comparative observational | 33 | 35 ± 10 | Obesity | BIB/Orbera | 6 | AST, ALT, GGT | NR |
% EWL 29.2, % EBL 29.3 18/33 (54.5%) achieved weight loss of ≥10% |
| Stimac 201152 | Non comparative observational | 165 | 39 ± 11 | Obesity | BIB/Orbera | 6 | ALT, GGT | NR | % EWL 39.7 ± 23.6, % EBL 39.5 ± 25.1 |
| Lee 20127 | RCT (IGB vs. Sham) | 8 (BIB) vs. 10 | 43 ± 20 | Obesity + NASH | BIB/Orbera | 6 | AST, ALT, NFS; median NAS was significantly lower in the IGB group vs. sham group: 4 vs. 2 | Liver biopsy | |
| Zerrweck 201244 | Comparative observational; gastric bypass with pre-op IGB vs. gastric bypass only | 23 | 44 ± 11 | Obesity | BIB/Orbera | 6 | ALT, GGT | NR | Pre-Op weight loss with IGB prior to laparoscopic gastric bypass in super-super obese pts |
| Tai 201353 | Non comparative observational | 28 | 32 ± 9 | Obesity | BIB/Orbera | 6 | AST, ALT | NR |
Median % EWL 40.1 in 28/28 20 pts (71.4%) lost >20% EWL (responders) |
| Folini 201441 | Comparative observational (vs. diet/exercise) | 18 (5 LAGB, 13 IGB vs. 13 diet) | 43 ± 12 | Obesity | BIB/Orbera | 6 | ALT, GGT; liver steatosis by chemical shift MRI, significant ↓ in Weight, BMI and ALT/AST | Ultrasound, MRI | NR |
| Takihata 201442 | Observational, prospective, comparative IGB vs. intensive LM | 8 (BIB) vs. LM (8) | 40.9 ± 13.9 | Obesity | BIB/Orbera | 6 | CT liver volume: liver volume reduced from 2086 ± 576 to 1793 ± 589 after 6 months | CT scan | % EWL in the IGB group: 65.4 ± 20.2 to 54.2 ± 21.5 |
| Majanovic 201443 | Prospective comparative (vs. CBT) | 60 (IGB) vs. 54 (CBT) | 38.6 ± 11.0 | Obesity | BIB/Orbera | 6 | ALT, GGT | NR | % EWL = 44.6 ± 23.9 |
| Raftopoulos 201754 | Non comparative observational | 11 | 41 | Obesity | Ellipse, swallowable | 4 | ALT, AST | NR |
% Mean EWL 50.2% % TWL 14.6% |
| Nguyen 201755 | Non comparative observational, Retrospective |
135 67 = 1 IGB 48 = 2 IGBs 20 = 3 IGBs |
47.1 ± 12.2 | Obesity + NAFLD | BIB/Orbera | 6 | AST, ALT | NR |
67/135: 22.5% EWL Highest weight loss seen in 1st 6 months after Tx with 1 IGB |
| Bazerbachi 202156 | Non comparative observational | 21 | 54 ± 8 | Obesity + NASH + early fibrosis | Orbera | 6 |
AST, ALT, APRI, MRE, NAS improved in 90% (18/20) from median of 4 patients to 1 patient. Fibrosis improved in 3/20 |
Liver biopsy, MR elastography | % Mean TBWL = 11.7 ± 7.7% |
| Salomone 202157 | Non comparative observational, retrospective | 26 | 53 | Obesity + NASH + fibrosis | Orbera | 6 | Reduction of liver stiffness score from 13.3 ± 3.2 to 11.3 ± 2.8; Reduction of CAP score, FIB-4, AST and ALT | Transient elastography (fibroscan), Ultrasound |
Significant TBWL, 106 ± 19.7 to 92 ± 18.3 kg, p < 0.001 16/26 achieved TBWL > 10% 10/26 achieved TBWL: 7%–10% |
| Vijayaraghavan 202258 | Non comparative, observational | 56 | 53.8 ± 10.33 | Obesity + NASH Cirrhotic | Spatz Adjustable | 6 | Non-significant decrease in ALT/AST, Significant ↓ in CAP (10.09% reduction), Non-significant ↓ in LSM—mean reduction of 7.86 kPa, Change in HVPG of 11.12% | US, Transient Elastography (Fibroscan) |
Mean TBWL of 15.88 kg %TBWL of 16.46% Mean change in BMI of 10.1% % TBWL of ≥10% was achieved in 31 patients (55.35%) |
| Kessler 202259 | Non comparative observational | 12 | 37.36 ± 3.63 | Obesity | BIB | 6 | Significant ↓ in the volume of left liver lobe from 394 ± 39.27 mL to 353.4 ± 27.68 mL | CT scan | Significant reduction in BMI: mean BMI reduced from 52.51 ± 2.35 to 46.92 ± 1.90 |
- Abbreviations: ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; BIB, bioenteric intragastric balloon; BMI, body mass index; EWL, excess weight loss; GGT, gamma glutamyl transferase; LSM, liver stiffness measurement; MRE, MR elastography; TBWL, total body weight loss.
| Study | BMI | WEIGHT | AST | ALT | GGT | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Proc | Post-Proc | Pre-Proc | Post-Proc | Pre-Proc | Post-Proc | Pre-Proc | Post-Proc | Pre-Proc | Post-Proc | |
| Frutos 2007 | 55.2 ± 6.9 | 47.4 ± 7.7 | 149.3 ± 26.3 | 128 ± 20.1 | NR | NR | NR | NR | NR | NR |
| Ricci 2008 | 42.1 ± 5.8 | 37.8 ± 5.5 | NR | NR | NR | NR | 31.5 (10–126) | 24 (9–73) | 31 (7–106) | 23.5 (6–82) |
| Donadio 2009 | 44.8 ± 8.9 | 38.9 ± 6.8 | 122.2 ± 25.9 | 104.2 ± 22.1 | NR | NR | 30.7 ± 14 | 23.4 ± 9.3 | 29.8 ± 19.1 | 28.0 ± 28.1 |
| Forlano 2010 | 43.1 ± 8 | 38.0 ± 8 | 118 ± 24 | 101 | NR | NR | 39.3 ± 25.6 | 24.4 ± 10 | 37.5 ± 20.5 | 24.5 ± 17.1 |
| Sekino 2011 | 44 | 41.2 | 117.6 | 110.7 | 33.5 (9–72) | 19 (14–67) | 52.5 (10–182) | 25 (15–161) | 47 (27–107) | 34 (23–74) |
| Nikolic 2011 | 41.4 | 35.6 | 114 | 103 | NR | NR | 30 (8–101) | 27 (4–71) | 31 (9–212) | 21 (9–156) |
| Stimac 2011 | 41.6 ± 7.5 | 35.8 ± 7.9 | 123.2 ± 27.1 | 106.3 | NR | NR | 34.7 ± 31.5 | 26.5 ± 23.1 | 33.3 ± 23.3 | 24.7 ± 16.9 |
| Lee 2012 | 30.3 ± 5.7 | 28.7 ± 8.1 | NR | NR | 74.5 ± 46.75 | NR | 97 ± 34 | NR | NR | NR |
| Zerrweck 2012 | 65 ± 3.8 | 60.5 ± 4.3 | 178.6 ± 15.8 | 166.5 ± 16.6 | NR | NR | 43.8 ± 31.9 | 29.1 ± 13.5 | 65.8 ± 52.9 | 41.1 ± 31.5 |
| Tai 2013 | 32.4 ± 3.7 | 28.5 ± 3.7 | NR | NR | NR | NR | 49 (15–196) | 22 (6–99) | NR | NR |
| Folini 2014 | 43.8 ± 6.62 | 38.2 ± 6.19 | NR | NR | 22.2 ± 4.36 | 16.1 ± 2.99 | 25.9 ± 10.31 | 18.1 ± 5.96 | 27.8 ± 27.57 | 17.9 ± 12.21 |
| Takihata 2014 | 45.2 ± 5.9 | 41 ± 6.2 | 127.1 ± 24.4 | 115.9 ± 26.4 | 32.4 ± 20.1 | 25.5 ± 17.5 | 57.1 ± 55.6 | 43.1 ± 48.8 | 53 ± 25.4 | 40.1 ± 19.3 |
| Majanovic 2014 | 38.6 ± 3.9 | 32.8 ± 4.3 | 113.8 ± 17.9 | 97.2 ± 17.7 | NR | NR | 31.1 ± 17.4 | 23.5 ± 10.6 | 33.3 ± 19.6 | 25.8 ± 14.4 |
| Raftopoulos 2017 | 36.1 ± 3.2 | 30.7 ± 4.0 | 103.5 ± 15.8 | 88.1 ± 16.3 | 24.3 ± 9.96 | 15.7 ± 4.54 | 35.54 ± 23.52 | 15.27 ± 6.32 | NR | NR |
| Nguyen 2017 | 41.7 | 37.6 | 117.9 | 106.6 | 35.1 ± 25.2 | 32.8 | 38.9 ± 30.6 | 31 | 62.6 ± 74.9 | 39.1 |
| Bazerbachi 2021 | 43.2 ± 6.8 | 37.9 ± 6.6 | 122.3 ± 26.4 | 107.9 ± 24.9 | 67.5 ± 48.8 | 31.32 ± 20 | 91.6 ± 59.9 | 39.4 ± 25.4 | NR | NR |
| Salomone 2021 | 35.1 ± 4.7 | NR | 106 ± 19.7 | 92 ± 18.3 | 72.1 ± 40.3 | 34.3 ± 22.4 | 84.5 ± 42.3 | 46.7 ± 24.6 | 136 ± 51 | 94 ± 62 |
| Vijayaraghavan 2022 | 35.24 ± 3.92 | 32.19 ± 4.05 | 96.46 ± 15.01 | 80.58 ± 14.93 | 50.70 ± 33.76 | 38.05 ± 18.98 | 36.93 ± 27.54 | 27.95 ± 14.25 | NR | NR |
| Kessler 2022 | 52.51 ± 2.35 | 46.92 ± 1.90 | NR | NR | NR | NR | NR | NR | NR | NR |
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma glutamyl transferase; Proc, procedure; NR, not recorded.
3.2 Primary outcomes
3.2.1 NAFLD activity score (NAS)
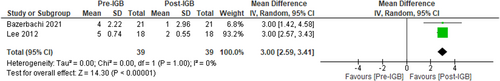
Two studies,7, 56 (n = 29, I2 = 0%) assessed the impact of IGB therapy on NAS following histological assessment of liver biopsy samples. NAS reduced significantly, MD: −3 [95% CI: 02.59 to −3.43, p < 0.01] following 6 months IGB therapy, favouring the use of IGB (Figure 2). Substantial heterogeneity was not found.
3.2.2 Liver enzymes
Sixteen studies evaluated the impact of IGB therapy on ALT, 8 studies on AST and 12 studies on GGT. All studies showed a significant reduction in liver enzymes.
Alt
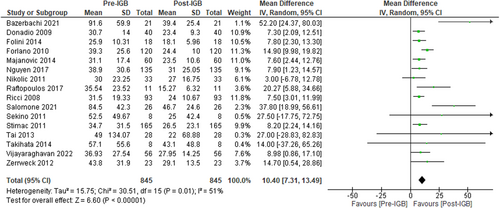
Sixteen studies (n = 845, I2 = 51%)41-44, 47-58 evaluated the effect of IGB therapy on serum ALT and showed a significant reduction in ALT level following 6 months of IGB therapy, MD: −10.40 U/L [95% CI: −7.31 to −13.49, p < 0.01] as seen in Figure 3.
AST
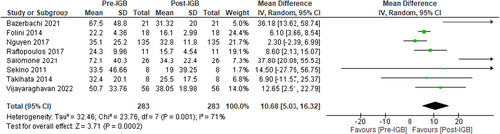
Eight studies (n = 283, I2 = 71%)41, 42, 50, 54-58 evaluated the effect of IGB therapy on serum AST.
These studies showed a significant reduction in AST level, MD: −10.68 U/L [95% CI: −5.03 to −16.32, p < 0.01] as shown in Figure 4.
GGT
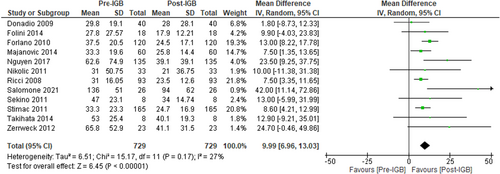
Twelve studies (n = 729, I2 = 27%)41-44, 47-52, 55, 57 evaluated the impact of IGB therapy on serum GGT level and showed a significant reduction, MD: −9.99 U/L [95% CI: −6.96 to −13.03, p < 0.01] (Figure 5).
3.3 Secondary outcomes
3.3.1 Liver volume
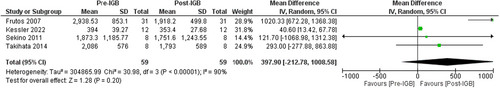
Four studies (n = 59, I2 = 90%)42, 46, 50, 59 evaluated the impact of IGB on liver volume by using Imaging. These studies showed a non-significant reduction in liver volume, MD: −397.90 [95% CI: −212.78 to −1008.58, p = 0.20] as seen in Figure 6.
3.3.2 Liver steatosis
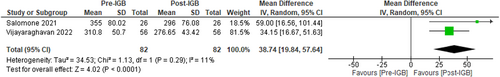
Using control attenuated parameter (CAP) via Vibration Controlled Transient Elastography (FibroScan) to measure the impact of IGB therapy on liver steatosis, two studies (n = 82, I2 = 11%)57, 58 showed a significant median difference in CAP scores of −38.74 dB/m [95% CI: −19.84 to −57.64, p < 0.01] favouring IGB therapy as seen in Figure 7.
Furthermore, The study by Folini et al 2014 (n = 18)41 showed a significant reduction in hepatic fat fraction via chemical shift MRI (16.7 ± 10.91 to 7.6 ± 9.76, p = 0.003), while the study in 2010 by Forlano et al (n = 120),49 also showed significant reduction in hepatic steatosis (assessed by ultrasound) from 52% to 4% (p < 0.0001).
3.3.3 Liver fibrosis
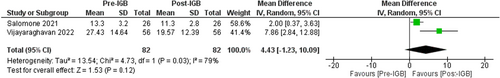
The impact of IGBs on liver fibrosis was evaluated by liver stiffness measurement (LSM) in Kilopascals via Fibroscan in two studies (n = 82, I2 = 79%)57, 58 which showed a non-significant decline in LSM, MD: −4.43 [95% CI: −1.23 to −10.09, p = 0.12] as seen in Figure 8.
Bazerbachi et al (n = 22)56 used AST-to-platelet ratio index (APRI) and magnetic resonance elastography (MRE) to assess liver fibrosis and showed that IGB therapy resulted in a significant decrease in APRI by 0.73 (p = 0.005) and magnetic resonance elastography-detected liver stiffness by 0.3 KPa (p = 0.03).
3.3.4 Glycated haemoglobin (Hba1c)
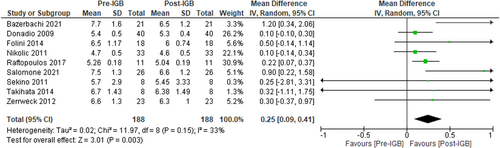
Nine studies (n = 188, I2 = 33%)41, 42, 44, 48, 50, 51, 54, 56, 57 evaluated the impact of IGB on HBa1c. These studies showed a mean reduction in HBa1c, MD: −0.25 [95% CI: −0.09 to −0.41, p < 0.01] as shown in Figure 9.
3.3.5 BMI & total body weight loss (TBWL)
Eighteen studies (n = 888, I2 = 0%)7, 41-44, 46-49, 51-59 evaluated the effect of IGB therapy on BMI. These studies showed a significant reduction, MD: −4.83 [95% CI: −4.31 to −5.36, p < 0.01] (Figure 10A).
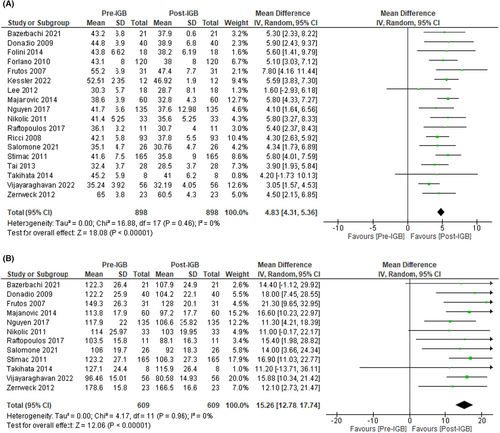
In addition, 12 studies (n = 609, I2 = 0%)42-44, 46, 48, 51, 52, 54-58 showed a substantial decline in total body weight loss, MD: −15.26 [95% CI: −12.78 to −17.74, p < 0.01) (Figure 10B).
3.3.6 Insulin resistance
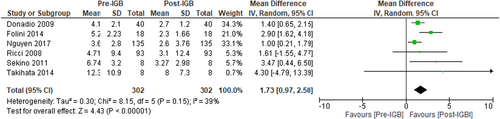
Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was used to evaluate the effect of IGB therapy on insulin resistance. Six studies (n = 302, I2 = 39%)41, 42, 48, 49, 51, 56 evaluated the impact of IGB therapy on HOMA-IR and showed a significant decline in insulin resistance, MD: −1.73 [95% CI: −0.97 to −2.50, p < 0.01] (Figure 11).
4 SAFETY AND DURABILITY
Most of the included studies reported data on adverse events following IGB placement. The most common reported symptoms were nausea, vomiting, abdominal discomfort and reflux symptoms. These were in most cases mild, transient and resolved with medications. 11 studies provided data on premature IGB removal—56 patients (6.14%); this occurred due to intolerance mostly as a result of persistent nausea, vomiting and abdominal pain. Other reasons included reflux symptoms, panic attack, voluntary decision, psychological intolerance and loss of early satiety. Five studies reported data on complications, overall, there were 3 cases of gastric outlet obstruction (0.3%), 2 cases of spontaneous balloon deflation (0.2%) and 2 cases of emergency hospital admission due to intractable nausea and vomiting resulting in dehydration and Acute Kidney Injury (0.2%). There were no reported deaths in any of the studies (full details in Table S1). In a meta-analysis which pooled 15 studies and included 3608 patients, there was an early balloon removal rate of 4.2%, 26 obstructions in the GI tract and 4 perforations.60
5 DISCUSSION
This systematic review and meta-analysis pooled the results of studies evaluating the effect of Intragastric Balloon (IGB) on NAFLD. The meta-analysis evaluated liver-related outcomes including Liver enzymes, NAFLD activity score (NAS), Liver volume, Liver steatosis and Liver fibrosis; as well as non-liver-related outcomes such as Body weight, BMI, Insulin resistance and glycated haemoglobin (HBa1c). It showed that IGBs can induce significant weight loss, subsequently leading to improvement in major NAFLD surrogates. Overall, there was significant improvements in some of the pooled liver-related outcomes, as well as improvements in surrogate markers of insulin resistance and glycated haemoglobin. IGBs also help downregulate ghrelin, delay gastric emptying and increase circulating SIRT-1 action.61
Intragastric balloons and other forms of EBTs have shown the potential to bridge the gap that exists between non-surgical and surgical treatment for obesity, and by consequence, NAFLD. In a meta-analysis by Chandan et al34 on the efficacy of Intragastric Balloons in NAFLD, involving 9 studies and 452 patients, improvements were observed in steatosis (79.2%), NAS score (83.5%) and HOMA-IR (64.5%). In addition, a reduction of liver volume was observed in most patients (94%). Furthermore, a similar meta-analysis by Freitas Junior et al62 including 10 studies and 508 patients, which also focused on IGBs, showed improvement in liver enzymes and metabolic markers related to NAFLD progression. Our meta-analysis in comparison, the most comprehensive to date, included 19 studies and 911 patients and showed improvements in NAFLD Activity score (NAS), liver enzymes, liver volume, HOMA-IR, total body weight loss, glycated haemoglobin and BMI. It is important to note that while IGBs may offer therapeutic potential in NAFLD, they are temporary devices; there is insufficient data regarding long-term maintenance of weight lost following removal of an IGB. In the 33 patients who completed Mathus-Vlegen et al's study,63 weight loss was 25.6 kg (20.5%) after 1 year of IGB therapy, this reduced to 14.6 kg (11.4%) 12 months after balloon removal.
Regarding safety, most patients do experience adverse events in the early days after IGB placement albeit most are transient and mild. In addition, complications have also been reported, although rare. In order to reduce the rate of early balloon removal due to intolerance, the use of gastric emptying studies has been suggested to detect gastroparesis. Lopez-Nava et al's study,64 which involved 32 patients, concluded that utilising baseline gastric emptying to predict intolerance to IGB may have prevented 75% of early removal cases.
Apart from IGBs, other EBTs have shown promise in the treatment of NAFLD in small studies, as shown by Hajifathalian et al30 with Endoscopic Sleeve Gastroplasty (ESG), Al Khatry et al with POSE, Gollisch and Karlas with duodenal-jejunal bypass liner and Van Baar with duodenal mucosal resurfacing. Moreover, Ren et al's35 recent meta-analysis concluded that EBTs could potentially ameliorate NAFLD based on the evidence of improved liver steatosis, liver function and insulin resistance.
This meta-analysis provides an in depth and up-to-date review of the impact of IGB on NAFLD and associated metabolic parameters and adds to the depth of existing literature on the efficacy of IGB as a treatment tool in NAFLD. It highlights a paucity of high-quality data on this subject, for example, only 4 of the included studies evaluated the effect of IGB on liver fibrosis. Lee et al7 conducted a RCT involving eighteen patients (8 in the IGB group and 10 in the sham group) to evaluate the effect of IGB on NASH. They showed that the overall NAS score (sum of histologic steatosis, ballooning and inflammation scores), was significantly lower in the IGB group versus the sham group. However, changes in individual components, that is steatosis, ballooning, inflammation and fibrosis scores, were not significant (possibly limited by small study numbers).
More recently, in its single-arm study involving 29 patients with early liver fibrosis, who underwent MR Elastography and EUS-guided liver biopsy at the time of IGB placement and removal, Bazerbachi et al56 showed a significant reduction in mean TBWL, HBa1c and waist circumference. NAS score improved in 90%, with a median decrease of 3 points. In regard to liver fibrosis, no changes were observed in 12 patients, 5 patients showed deterioration, with improvement in 3 patients. Salomone et al57 retrospectively assessed the effects of IGB in a cohort of 26 obese patients with liver stiffness scores ≥9.7 KPa, measuring changes in metabolic and liver parameters. They observed a reduction of liver stiffness measurement and FIB-4 scores 6 months after removal of IGB. Vijayaraghavan et al58 showed the effect of IGB on obese NASH compensated cirrhotic patients. Apart from achieving a significant weight reduction of 15.88 kg (16%), there was also a mean reduction of liver stiffness measurement of 28.6%, as well as a reduction of Hepatic venous pressure gradient. All of these studies had small sample sizes, and only one of them was a RCT. In addition, in about half of the included studies, although reduction of liver enzymes was observed, a formal diagnosis of NAFLD was not documented.
In relation to the existing knowledge gap regarding the use of IGBs in the management of NAFLD, the impact of weight loss induced by IGB on validated serum biomarkers of fibrosis in NAFLD have not yet been studied. These biomarkers include – tissue inhibitor of matrix metalloproteinases-1 (TIMP-1), amino terminal propeptide of procollagen type III (P3NP) and hyaluronic acid (which combined make up the ELF Panel—Enhanced Liver Fibrosis Panel) and other inflammatory cytokines that are potentially involved in the fibroinflammatory cascade which occur in patients with NAFLD.
5.1 Strengths and limitations
A key strength of this meta-analysis is its inclusion of studies that evaluated liver-related outcomes as primary or secondary endpoints, as well as non-liver-related outcomes—weight reduction and metabolic parameters. A large proportion of the existing literature on the therapeutic use of IGB focuses on obesity and weight reduction. This study hence adds to the existing literature on the efficacy of IGB on NAFLD-related parameters.
There are a number of limitations, and they include (1) Small sample size of most of the included studies (2) Only one randomised controlled trial was included (3) The majority of the studies included focused on weight loss as their primary endpoint, with liver-related outcomes analysed as secondary endpoints. Hence, they may not be appropriately powered to detect changes in NAFLD surrogate markers. (4) Only half of included studies, provided data on the diagnosis of NAFLD and the modality employed. The remaining studies used elevated liver enzymes as an indirect measure of NAFLD diagnosis. Furthermore, data on key NAFLD indices including fibrosis and steatosis were lacking in most studies. This does show the dearth of data that exist on the use of IGB as a therapeutic tool in NAFLD.
Despite these limitations, and despite IGBs being temporary devices, Intragastric balloon therapy may yet play a role in the management of NAFLD going forward, especially given the limited treatment options available. IGBs can kickstart a weight loss journey, which in combination with lifestyle modification, can reverse disease progression in NAFLD/NASH. Maintaining weight loss after removal of IGBs is a valid concern, however, continued adherence to modified lifestyle changes, along with the use of weight loss medications, that is GLP1-receptor agonists could help prevent weight regain.65 More high-quality studies are needed to explore this.
6 CONCLUSION
Intragastric balloon therapy appears to be an effective treatment option to induce significant weight loss in obese patients. This review and meta-analysis highlights its potential use in the treatment of obese patients with NAFLD. Induction of weight loss via these devices can lead to improvements in liver-related outcomes, as well as metabolic parameters. This can potentially bridge the gap in the management of these patients, especially those with established fibrosis who are at risk of progression to liver cirrhosis. However, large-scale long-term studies are required before IGBs can be recommended as a treatment tool for patients with NAFLD.
AUTHOR CONTRIBUTIONS
Olufemi Aoko: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; validation; visualization; writing – original draft; writing – review and editing. Tobias Maharaj: Data curation; formal analysis; methodology; project administration; writing – review and editing. Fiona Boland: Formal analysis; writing – review and editing. Danny Cheriyan: Conceptualization; funding acquisition; project administration; resources; supervision; validation; visualization; writing – review and editing. John Ryan: Conceptualization; funding acquisition; project administration; resources; supervision; validation; visualization; writing – review and editing.
ACKNOWLEDGEMENTS
Declaration of personal interests: We thank Breffni Smith and Andrew Simpson, who are clinical librarians at Beaumont Hospital Dublin affiliated with the Royal College of Surgeons in Ireland (RCSI), for providing valuable input in designing the search strategy for this review. Open access funding provided by IReL.
FUNDING INFORMATION
Charitable Infirmary Charitable Trust—Kieran Taaffe Bursary; Beaumont Hospital/RCSI Dublin.




