Blood basophils and asthma among participants from CONSTANCES, the French population-based cohort
Along with eosinophils and neutrophils, basophils have initiator and effector functions in allergy and inflammation.1 However, their role in asthma remains largely unexplored. In adults from CONSTANCES, a population-based cohort, blood inflammatory phenotypes based on eosinophils and neutrophils were associated with distinct clinical expressions of asthma.2 In the present study, we hypothesized that basophils provide additional information to disentangle the heterogeneity of asthma in the same cohort (https://www.constances.fr/index_EN.php).
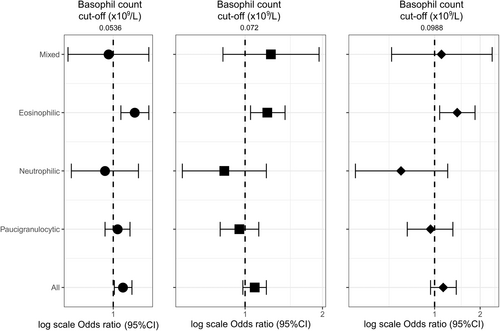
At baseline, adults with data on ever-asthma and white blood cell counts were included (n = 161,796, Figure S1). Four years later, 2896 participants were followed-up, including 1223 with CA (Figure S1, Tables 1 and S1). Current asthma (CA) was defined by asthma attacks, symptoms, or treatments in the last 12 months. Blood eosinophil and neutrophil cut-offs defined paucigranulocytic, neutrophilic, eosinophilic, and mixed inflammatory phenotypes.2 The 75th percentile of the basophil distribution among the 161,796 participants was defined as a cut-off. Regression models adjusted (a) for age, sex, smoking status, education level, French deprivation index (Fdep), treatment, and body mass index were used (main model), and several supplementary analyses were performed (Supporting information).
| At baseline n = 15,206 | At follow-up n = 2896 | |
|---|---|---|
| Age, year, mean ± SD | 44.4 ± 13.6 | 51.7 ± 13.1 |
| Sex, women, n (%) | 8604 (56.6) | 1583 (54.7) |
| Smoking habits, n (%) | n = 14,702 | n = 2582a |
| Never smokers | 6654 (45.3) | / |
| Ex-smokers | 4843 (32.9) | / |
| Current smokers | 3205 (21.8) | 237 (9.2) |
| Body Mass Index (BMI), kg/m2, n (%) | n = 15,076 | |
| <18.5 | 403 (2.70) | 741 (25.6) |
| 18.5–25 | 7417 (49.2) | 1093 (37.7) |
| 25–30 | 4770 (31.6) | 681 (23.5) |
| ≥30 | 2486 (16.5) | 381 (13.2) |
| Education level, n (%) | n = 15,040 | n = 2872 |
| Lower than high school | 3575 (23.8) | 565 (19.7) |
| High school | 2553 (17.0) | 431 (15.0) |
| College | 4168 (27.7) | 834 (29.0) |
| University | 4744 (31.5) | 1042 (36.3) |
| FDep, n (%) | n = 15,025 | n = 2.896 |
| FDep quintile 1, less deprived | ||
| 3015 (19.8) | 582 (20.1) | |
| FDep quintile 2 | 2984 (19.6) | 577 (19.9) |
| FDep quintile 3 | 3104 (20.4) | 612 (21.1) |
| FDep quintile 4 | 3048 (20.1) | 545 (18.8) |
| FDep quintile 5 | 3054 (20.1) | 580 (20.0) |
| Basophil cut ≥0.0536 × 109/L | 4320 (28.4) | 371 (30.3) |
| Asthma attacks (last 12 months), n (%) | n = 14,155 | n = 2397 |
| 5404 (38.2) | 676 (28.2) | |
| Asthma symptom score, n (%) | n = 14,264 | n = 2761 |
| 0 | 2095 (14.7) | 1138 (41.2) |
| 1 | 4843 (34.0) | 685 (24.8) |
| 2 | 3269 (22.9) | 431 (16.7) |
| 3 | 1916 (13.4) | 258 (9.3) |
| 4 | 1223 (8.60) | 126 (4.6) |
| 5 | 918 (6.40) | 93 (3.4) |
| Nocturnal symptoms (last 12 months), n (%) | ||
| Woken with chest tightness | n = 14,989 | n = 2836 |
| 6546 (43.7) | 831 (29.3) | |
| Woken by an attack of shortness of breath | n = 14,929 | n = 2839 |
| 2240 (15.0) | 269 (9.5) | |
| Woken by an attack of coughing | n = 14,993 | n = 2833 |
| 6145 (41.0) | 784 (27.7) | |
| Any asthma treatment, n (%) | n = 14,698 | n = 2427 |
| 7052 (48.0) | 1067 (44.0) | |
- Abbreviations: Fdep, the French deprivation index; SD, standard deviation.
- a The question has changed to “Do you currently smoke (excluding electronic cigarettes)? Yes (at least once a day) or No or occasionally”.
Among the 161,796 participants, basophil count ranged from 0.0027 to 0.43 × 109/L and differed by asthma status ((a)means: 0.0431, 0.0438, and 0.0456 × 109/L in never-, non-current and CA respectively, ptrend < 10−4). Basophil count ranged from 0.0027 to 0.43 × 109/L among never-asthmatics (median [Q1–Q3] of 0.038 [0.0258–0.0531] × 109/L), and from 0.0029 to 0.301 × 109/L among current asthmatics (0.0402 [0.028–0.057] × 109/L, Figure S2, Kuiper's test for homogeneity of distributions, p < 10−4). Among participants with CA (n = 15,206, Table 1), basophil count increased with age, and differed according to smoking, Fdep and treatment (Table S2). Both at baseline and at follow-up, significant correlations were found between basophil and eosinophil ((a) r = 0.30/0.39 at baseline/follow-up) and neutrophil ((a) r = 0.19/0.20) counts, and basophil count differed according to inflammatory phenotype (Table S3, p < 10−4). At follow-up, 83.7% of the participants with low (n = 857) and 63.1% of the participants with high (n = 366) basophil count at baseline were in the same basophil class (κ = 0.466 95%CI [0.412–0.519]). At baseline, high basophil count was associated with a higher risk of woken by an attack of cough, and specifically among those with the eosinophilic phenotype (Figure 1). Results did not change whatever the cut-off used or after excluding participants with chronic bronchitis (Table S4), after additional adjustments or in sensitivity analyses (Table S5). No associations were found with the other asthma characteristics (not shown). At follow-up, participants with high basophil count at baseline were at higher risk of having eosinophilic and mixed phenotypes, CA, and woken with chest tightness (Figure 2), and associations persisted after adjustment on eosinophil count, additional adjustments or in sensitivity analyses (Table S6).
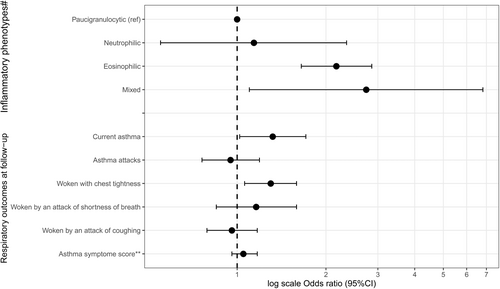
This study is the first to explore blood basophils levels in association with clinical and biological characteristics of asthma and their evolution among adults with non-severe asthma from a large population-based cohort. We acknowledge that assessing asthma by questionnaire could be considered as a weakness, and we do not exclude some bias selection due to the lost to follow-up participants. We also acknowledge that the technique used to measure basophils may be viewed as a limitation. The analytical performance of the analyzers used routinely in laboratory medicine for measuring basophils is lower than that of analyzers using flow cytometry, leading to under or over estimation of basophil counts with some analyzers due to the presence of pathological leukocytes.3 The CONSTANCES population does not include, or only marginally, individuals with serious hematological diseases, and we applied a two-step procedure to be as specific as possible to capture participants with asthma. The lack of skin prick tests and immunoglobulin E measurements did not allow to investigate associations between basophils and allergic asthma. We found increased blood basophil count in asthmatics as compared to never-asthmatics and correlations between basophil and eosinophil counts that agree with two previous studies that investigated sputum basophils in asthmatic patients compared with healthy controls.4, 5 Increased risks of subsequent eosinophilic/mixed asthma and CA among participants with high basophil counts at baseline, suggest more active T2-oriented disease. Increased frequency of nocturnal cough and woken with chest tightness might reflect a specific clinical phenotype or poorer disease control. Accordingly, children with acute asthma and high blood basophil counts have been shown to be more likely to experience additional exacerbations during the follow-up.6 In conclusion, even if these results warrant further investigations, they suggest an actual role of basophils as actors and/or potential biomarkers in asthma.
AUTHOR CONTRIBUTIONS
JH, NR, and RN designed and conducted the research; CR, MG, and MZ provided essential materials; RN, TT, and LO analyzed data and performed statistical analyses; RN wrote the manuscript and had primary responsibility for final content; all authors read, edited, and approved the final manuscript.
ACKNOWLEDGMENTS
The authors thank the “Caisse nationale d'assurance maladie” (CNAM) and the “Centres d'examens de santé” of the French Social Security which are collecting a large part of the data, as well as the “Caisse nationale d'assurance vieillesse”, ClinSearch, Asqualab and Eurocell in charge of the data quality control. The authors thank all those who participated to the setting of the study and on the various aspects of the examinations involved: interviewers, technicians for lung function testing, coders, those involved in quality control, data and sample management and all the staffs from the inclusion centres (HPCs). They are indebted to all the participating individuals without whom the study would not have been possible. The authors also thank S Le Got, S Lemonnier, A Ozguler, C Ribet from Inserm UMS11. The authors are also grateful to the “Groupe Respiratoire CONSTANCES”: MC Delmas, O Dumas, V Giraud, Y Iwatsubo, B Leynaert, N Le Moual, R Nadif, T Perez, N. Roche, R Varraso.
FUNDING INFORMATION
The Constances cohort study was supported and funded by the French national health insurance fund (« Caisse nationale d’assurance maladie », Cnam). Constances is an National infrastructure for biology and health (“Infrastructure nationale en biologie et santé”) and benefits from a grant from the French national agency for research (ANR-11-INBS-0002). Constances is also partly funded to a small extent by industrial companies, notably in the healthcare sector, within the framework of Public-Private Partnerships (PPP). None of these funding sources had any role in the design of the study, collection and analysis of data or decision to publish.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no relevant conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




