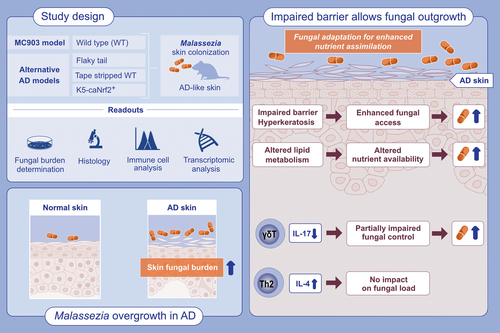
Epidermal barrier impairment predisposes for excessive growth of the allergy-associated yeast Malassezia on murine skin
Abstract
Background
The skin barrier is vital for protection against environmental threats including insults caused by skin-resident microbes. Dysregulation of this barrier is a hallmark of atopic dermatitis (AD) and ichthyosis, with variable consequences for host immune control of colonizing commensals and opportunistic pathogens. While Malassezia is the most abundant commensal fungus of the skin, little is known about the host control of this fungus in inflammatory skin diseases.
Methods
In this experimental study, MC903-treated mice were colonized with Malassezia spp. to assess the host–fungal interactions in atopic dermatitis. Additional murine models of AD and ichthyosis, including tape stripping, K5-Nrf2 overexpression and flaky tail mice, were employed to confirm and expand the findings. Skin fungal counts were enumerated. High parameter flow cytometry was used to characterize the antifungal response in the AD-like skin. Structural and functional alterations in the skin barrier were determined by histology and transcriptomics of bulk skin. Finally, differential expression of metabolic genes in Malassezia in atopic and control skin was quantified.
Results
Malassezia grows excessively in AD-like skin. Fungal overgrowth could, however, not be explained by the altered immune status of the atopic skin. Instead, we found that by upregulating key metabolic genes in the altered cutaneous niche, Malassezia acquired enhanced fitness to efficiently colonise the impaired skin barrier.
Conclusions
This study provides evidence that structural and metabolic changes in the dysfunctional epidermal barrier environment provide increased accessibility and an altered lipid profile, to which the lipid-dependent yeast adapts for enhanced nutrient assimilation. Our findings reveal fundamental insights into the implication of the mycobiota in the pathogenesis of common skin barrier disorders.
Graphical Abstract
The abundant commensal yeast Malassezia overgrows in AD-like skin. Using four different experimental models of AD, we show that Malassezia acquires enhanced fitness in the barrier-impaired and metabolically dysregulated skin. The lipid-dependent yeast adapts to the AD-like skin for enhanced nutrient assimilation.Abbreviations: AD, atopic dermatitis; WT, wildtype.
1 INTRODUCTION
The microbiota is an integral part of the skin ecosystem that enhances the barrier function of this essential organ ensuring protection of the host from dehydration and entry of harmful substances including toxins and pathogens.1, 2 To achieve this, skin commensal microbes educate defense and immune responses and contribute to proper differentiation and epithelialization. In turn, the disturbance of the microbial balance can compromise the physical, chemical, and immunological properties of the cutaneous barrier3, 4 and promote skin diseases characterized by barrier impairment, such as atopic dermatitis.5
The fungal community of the skin microbiota is unique in that it comprises mainly a single fungal genus, Malassezia, in humans as well as many warm-blooded animals.6 Of the 20 Malassezia species described so far, M. restricta and M. globosa dominate human skin, followed by M. sympodialis and M. furfur, while M. pachydermatis is the most abundant species on canine skin.7-9 Due to the lack of fatty acid synthase and the consequent dependence on exogenous lipids, Malassezia is highly adapted for life on the mammalian skin, where it finds favorable conditions in lipid-rich areas such as sebaceous hair follicles.10 Malassezia is tightly controlled by the cutaneous immune system, which by means of IL-17 mediated immunity prevents fungal overgrowth11-13 and thereby ensures homeostatic colonization.14 How immunosurveillance of the commensal fungus is affected under conditions of inflammation and barrier disruption in diseased skin is however not well understood. Malassezia-host interactions remain largely understudied, despite the fungus' dominance in the skin and its association with common skin pathologies, including seborrheic and atopic dermatitis, and more recently also with extracutaneous diseases including IBD15 and cancer.16, 17 In these diseases, Malassezia is thought to act as a pathogen and exacerbate inflammation. Irritant fatty acids and indoles produced by the fungus have been suggested to drive pathology. Still, the causative role of the fungus in disease remains largely unclear.
One of the most common skin disorders associated with Malassezia is atopic dermatitis (AD), a chronic relapsing skin disorder with an increasing incidence in both humans and dogs.18, 19 The skin of AD patients is characterized by barrier defects, an altered lipid metabolism, and immune dysregulation.18 Malassezia-specific IgE antibodies can be found in around half of all adult patients causing type I hypersensitivity.20 Allergic sensitization to Malassezia is most prominent in head- and neck dermatitis (HND), a difficult to treat subtype of human AD, for which an increased abundance of Malassezia on the skin has been observed.21, 22 Canine AD is also frequently accompanied by Malassezia-specific IgE antibodies23 and by Malassezia overgrowth in the ear canal and interdigital space.24, 25 While the direct contribution of Malassezia to AD pathogenesis remains to be determined, Malassezia has been reported to aggravate skin barrier impairment and to induce Th2 inflammation.21, 26 The fungus may facilitate inflammation and perpetuate damage to the impaired epithelial barrier integrity via lipases or proteases, which allow for microbial antigens and irritating metabolites to access deeper layers of the epidermis in an inflammatory amplifying loop.27, 28
To study how the diseased skin environment impacts the mutual interplay of Malassezia with the host, we employed four different experimental models reproducing important clinical features of skin barrier impairment and AD and assessed how changes in the immune status, barrier integrity and lipid metabolism affect Malassezia colonization. We provide evidence that Malassezia acquires enhanced fitness in the dysregulated skin. More specifically, we show that unrestrained Malassezia growth in the AD-like skin occurs due to epidermal permeability barrier dysfunctions, which provide better access to an altered niche. The fungus adapts to the alterations in lipid metabolism for enhanced lipid assimilation.
2 RESULTS
2.1 Malassezia grows excessively in the atopic skin environment
To study the interaction of Malassezia with the host under AD-like conditions, we adopted the widely used MC903 model in C57BL/6 mice.29, 30 Repeated application of the vitamin D analog calcipotriol (MC903) in comparison to solvent control to the murine ear skin for 10 days led to an increase in ear thickness (Figure 1A), epidermal hyperplasia (Figure 1B,C), elevated skin Tslp transcripts (Figure 1D) and serum IgE titers (Figure 1E). As previously described for the MC903 mouse model and characteristic for AD skin lesions,29 we observed an accumulation of diverse inflammatory immune cells (Figure S1). Epicutaneous application of M. pachydermatis31 to the dorsal side of the MC903 pretreated skin worsened epidermal hyperplasia (Figure 1B) without further increasing the overall ear thickness by day 4 and day 7 post-infection (Figure 1A,C). At these time points Tslp transcript levels were reduced in response to Malassezia association (Figure 1D). When comparing Malassezia-induced immune cell subsets in the MC903-treated skin to those in control-treated skin we found several known and suspected antifungal effector cell populations to be increased under inflamed skin conditions, including Vγ4+ and CD4+ T cells, and eosinophils (Figure S1A,B). Meanwhile, no Malassezia-induced cell populations were diminished in the skin of MC903-treated compared to control-treated mice (Figure S2). Of note, maintenance of the inflamed skin phenotype required continued application of MC903 (Figure 1A). To minimize interference of the ethanol solvent with fungal viability, the continuous treatment after infection was applied to the ventral side of the ear only (Figure 1A, Figure S2A–F). The unilateral treatment with MC903 led to a robust expression of the vitamin D receptor target gene Cyp24a1 on both dorsal and ventral ear sides and did not affect fungal loads or inflammatory infiltrates (Figure S2A–F).
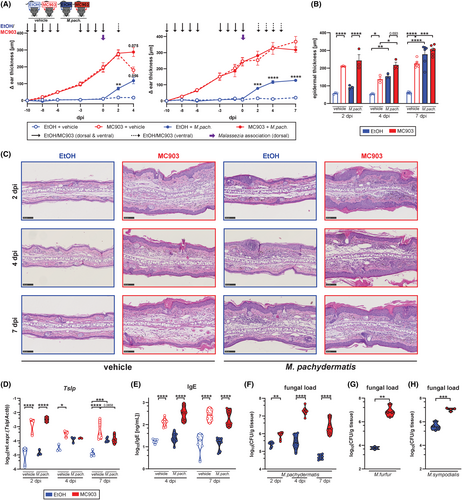
We then assessed the fate of Malassezia in the MC903-treated skin and found that the altered skin environment strongly interfered with fungal control. We observed pronounced fungal overgrowth in the MC903-treated skin compared to Malassezia-colonized control skin as early as 2 dpi with a further increase over the course of 7 days, while control mice steadily cleared the fungus (Figure 1F). The elevated fungal burden in MC903-pretreated skin was not limited to a single species of Malassezia but was also seen after infection with M. sympodialis and M. furfur (Figure 1G,H). Moreover, the same was observed in mice on the Balb/c background, which are widely used in allergy research due to their inclination to type 2 immune polarization (Figure S2G–I). Taken together, these data show that while Malassezia does not aggravate atopy in MC903-treated mice, it strongly overgrows under these conditions.
2.2 Malassezia overgrowth in the MC903-treated skin does not require type 2 cytokines
Given the pronounced induction of TSLP and other hallmarks of type 2 immunity in MC903-treated skin, we scrutinized TSLP for its contribution to Malassezia overgrowth. TSLP is the main driver of the type 2 response in the MC903 model.32, 33 Consequently, the number of overall IL-4+ cells as well as Th2 cells in the ear skin was increased upon MC903 treatment (Figure 2A), although the Th2 response was not directed against Malassezia in our experimental setup (Figure S3A–D). We thus made use of Tslpr−/− mice34 to assess the impact of TSLP and downstream effects on fungal control in response to MC903. Absence of TSLP signaling resulted in a decrease of overall ear inflammation after MC903 treatment as reflected by the diminished ear thickness (Figure 2B), the reduced number of inflammatory cells infiltrating the skin (Figure 2C, Figure S3E), and the lower serum IgE levels (Figure 2D), although disease was not completely abolished and manifested with an apparent ichthyosis phenotype (Figure 2C). Importantly, the lack of TSLP signaling had no effect on fungal control when comparing MC903-pretreated Tslpr−/− and wild-type (WT) mice (Figure 2E). These results were replicated in MyD88−/− mice lacking IL-1 family cytokine signaling including IL-33 and IL-18 signaling, which were reported to be involved in driving the MC903-induced type 2 skin inflammation.35, 36 In comparison to Tslpr−/− mice, MyD88-deficiency had little effect on the AD-like skin phenotype as indicated by the marked ear inflammation (Figure 2F,G) and elevated serum IgE levels (Figure 2H). Fungal overgrowth in response to MC903 was unchanged in comparison to MyD88-sufficient control animals (Figure 2I). Consequently, neither IL-1 family cytokine nor TLR signaling were responsible for the high fungal burden observed in the MC903-treated murine skin.
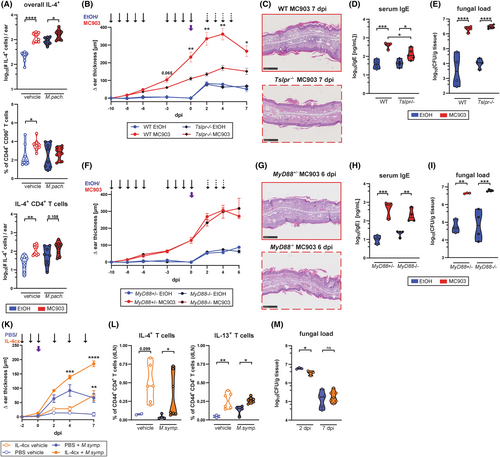
To further investigate the impact of type 2 immunity on fungal control in a MC903- and TLSP-independent context, we repeatedly injected WT mice with recombinant IL-4 to increase systemic IL-4 concentrations before and during skin colonization with Malassezia (Figure 2K). IL-4 was administered in the form of IL-4-anti-IL-4 monoclonal antibody complexes (IL-4cx), which prolongs the cytokine's in vivo biological half-life.37 While IL-4cx injection resulted in an increase in epidermal and overall ear thickness (Figure S3F) and percentages of IL-4- and IL-13-producing CD4+ T cells (Figure 2L), the fungal load was unaffected at both 2 and 7 dpi in comparison to the PBS-injected control group (Figure 2M). This indicates that systemic increase in type 2 cytokines did not interfere with fungal control and is therefore unlikely the cause of fungal overgrowth in the MC903 model of AD. Together, these results suggest that local or systemic type 2 cytokines are not directly responsible for impaired fungal control in the skin.
2.3 Decreased type 17 antifungal immunity alone does not explain Malassezia overgrowth in MC903-treated skin
IL-17 immunity is vital for antifungal defense in barrier tissues including cutaneous protection against Malassezia in experimentally colonized mice.14 We recently identified Vy4+ γδ T cells as the key mediators of protective immunity against Malassezia in the unperturbed skin (Ruchti et al., PLoS Pathogens in press, doi: 10.1101/2023.09.08.556784). Therefore, we aimed at assessing the role of IL-17 in fungal control in MC903-treated skin. We found reduced transcript levels of Il17a and Il17f at 4 and 7 days after M. pachydermatis association (Figure 3A). Bulk RNA sequencing of MC903-treated skin associated with M. pachydermatis and of corresponding controls revealed 341 upregulated genes at 7 dpi in the MC903 condition while only 44 genes were downregulated (FDR < 0.05, Log2 fold change >2) (Figure 3B,C). Among the downregulated genes, a strong reduction in the expression of fungus-induced Il17a and Il17f and other genes associated with IL-17-producing γδ T cells38 was observed. Consequently, cutaneous IL-17A producing yd T cells were reduced in skin and lymph nodes (Figure 3C, Figure S4A,B), while IFN-y-production, which was increased in response to MC903 treatment, was not affected by the presence of M. pachydermatis (Figure S4C). In line with previous reports on vitamin D and its analog calcipotriol (MC903) transcriptionally repressing the IL-17A locus and inhibiting accumulation of IL-17+ T cells in psoriasis and autoimmune encephalitis,39-43 we found that the decrease in Il17a transcript levels in the MC903-treated skin was independent of TSLPR or MyD88-signaling (Figure S4D,E).
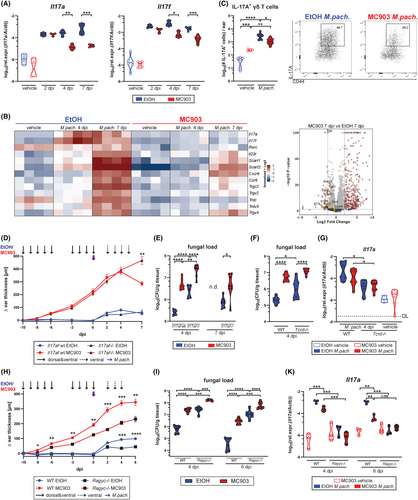
Next, we investigated whether MC903-dependent IL-17 repression was responsible for the observed fungal outgrowth in the AD-like skin (Figure 3D). IL-17A/F deficiency resulted in an increased fungal burden in control skin (Figure 3E), as we showed previously.14 This requirement for IL-17A/F in fungal control was also evident in the MC903-treated skin (Figure 3E). Importantly, fungal burden was increased in MC903- compared to ethanol control-treated skin, regardless of the presence or absence of IL-17A/F. Still, the fold difference in CFU was reduced in Il17af−/− mice (9-fold compared to 23-fold in WT mice), supporting a partial contribution of IL-17 repression to fungal overgrowth in the MC903-AD model (Figure 3E). These results were replicated in Tcrd−/− mice, lacking γδ T cell-derived IL-17 (Figure 3F,G), and in Rag2−/− Il2rg−/− (Ragγc−/−) mice, devoid of all lymphocyte and innate lymphoid cell (ILC) subsets (Figure 3H–K). Fungal overgrowth in Ragγc−/− mice was massive, while Il17a levels were generally very low in these mice, irrespective of treatment (Figure 3H–K). This confirms that neither IL-17- nor lymphocyte-mediated effects alone are responsible for the observed fungal overgrowth in the AD-like skin. Interestingly, we also found reduced Il17a levels in IL-4cx-treated mice for which fungal clearance was unaffected (Figure 2M, Figure S4F). Taken together, our results show that MC903-mediated repression of IL-17 can only partially explain Malassezia overgrowth in the atopic skin.
2.4 Physical barrier impairment in AD-like skin favors Malassezia overgrowth
We next considered epidermal barrier dysregulation, a main feature of AD,44 as an underlying cause of Malassezia overgrowth. The MC903 mouse model is characterized by physical epidermal barrier impairment as showed by increased TEWL and epidermal hyperplasia.45 This feature was preserved upon Malassezia association and conserved in all the immune deficient mouse lines manifesting fungal overgrowth upon MC903-treatment (Figure 4A). Consistent with these histopathological changes, keratinocytes were expanded, most prominently the subset of hair follicle keratinocytes, and displayed an activated phenotype (Figure 4B, Figure S5A–C). Of note, the stratum corneum including invaginating hyperkeratotic hair follicles are the main niche of Malassezia colonization in the skin (Figure 4C). This suggests that alterations in keratinocytes and especially the epidermal barrier function might underly Malassezia overgrowth upon MC903-induced inflammation.
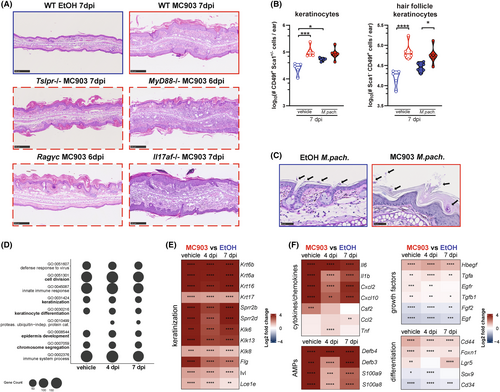
Scrutinizing our RNA-Seq dataset (Figure 4D–F, Figure S5D) revealed a strong response signature of epidermal barrier impairment and wound healing reminiscent of what has been described for lesional AD.46 Keratinization, epithelial cell division, and epidermis development were among the top differentially regulated pathways at all time points (Figure 4D–F). Importantly, all these MC903-induced changes were independent of the presence of Malassezia (Figure 4D–F). The high significance of the GO term “defense response to virus” appearing at the top of the list (Figure 4D) is explained by the strong induction of 2′-5′-oligoadenylate synthetases (OAS), which in addition to their antiviral activity also drive cell cycle progression and keratinocyte proliferation in the skin.47 Among the most highly upregulated genes were those involved in late keratinocyte differentiation, including involucrin (Ivl), filaggrin (Flg), and small proline-rich proteins (Sprr2b and Sprr2d),48 as well as the epithelium-specific serine proteases kallikreins, which are released from injured epithelium to promote desquamation49 (Figure 4E). The expression of inflammatory keratins (Krt6a, Krt6b, Krt16, and Krt17)50 was up-regulated as well (Figure 4E). Dense accumulations of keratohyalin granules containing keratin filaments, filaggrin, and loricrin, highlighted in H&E-stained sections, further illustrated the enhanced keratinization (Figure S5E). Given the close connection between re-epithelialization and the progression of wound healing, genes related to epidermal repair were differentially regulated in the MC903-treated skin, including Foxn1,51 and the hair follicle stem cell genes Lgr5, Sox9, and Cd3452 (Figure 4F). The epidermal and fibroblast growth factor genes Egf and Fgf2 were downregulated as observed in chronic wounds.53 The induction of key genes involved in glycolytic metabolism (Figure S5F) reflected the high energy demand of proliferating keratinocytes in lesional AD.54-56 Many of the inflammatory and antimicrobial genes that we found strongly induced under MC903 conditions are also linked to wound healing,46, 57, 58 including the small proline-rich proteins mentioned above that can exhibit antimicrobial function as shown recently.59 The strong antimicrobial and inflammatory signature in the MC903-treated murine skin (Figure 4F) was surprising considering the observed fungal overgrowth and suggested that Malassezia is able to overcome the host defense response. In summary, our results reveal little effects of innate and adaptive skin immunity on Malassezia overgrowth in the MC903-treated skin but highlights the overarching role of epidermal barrier impairment in fungal overgrowth in AD-like skin.
2.5 Fungal overgrowth is conserved across different models of epidermal barrier disruption
To assess the relevance of epidermal barrier impairment for fungal overgrowth beyond the MC903 model, we employed a model of genetically-induced epidermal barrier dysregulation owing to the constitutive activation of the antioxidant response factor Nrf2 in keratinocytes.60 Of note, Nfe2l2, the gene encoding Nrf2, as well as numerous Nrf2 response genes were upregulated in MC903-treated skin (Figure S5G). Hyperkeratosis and changes in the epidermal lipid envelope, resembling ichthyosis, in mice expressing a constitutively active Nrf2 mutant under the keratin 5 (K5) promoter (K5-caNrf2+ mice) is accompanied by strong upregulation of Krt16, the cornified envelope genes Slpi and Sprr2, the peroxisome proliferator activator receptor (Ppar) Ppard gene, as well as the long-chain fatty acid elongation factor Elovl4 and Elovl7 genes.60 These transcriptional changes in the skin of K5-caNrf2+ mice parallel the phenotype of MC903-treated skin (Figure S5F). Four days after skin association with M. pachydermatis, K5-caNrf2+ mice showed stable upregulation of Nrf2 and its target gene Nqo1 (Figure S6A,B) as well as genes involved in keratinocyte hyperproliferation and differentiation, such as Sprr2b and Krt16 (Figure 5A, Figure S6C). Most importantly, K5-caNrf2+ mice displayed a significant fungal overgrowth in the ear skin and even more pronounced at the snout compared to the corresponding control mice (Figure 5B). The exacerbated fungal load was accompanied by increased ear thickness and hyperkeratosis including accumulations of keratohyalin granules in comparison to cre-negative controls (Figure 5C,D, Figure S6D) pointing toward a disease-exacerbating role of the fungus.
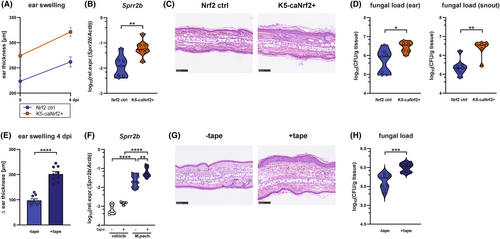
To further assess the relationship of skin barrier impairment with excessive fungal growth, we mechanically disrupted the epidermal barrier of mouse ears by tape stripping prior to association with Malassezia. Tape stripping elicits epidermal proliferation and hyperkeratosis as a consequence of the mechanical removal of the stratum corneum,61, 62 also reflected by increased keratohyalin granule formation (Figure S6E). Introduction of M. pachydermatis into this barrier-disrupted skin resulted in strongly exacerbated ear swelling, epidermal hyperplasia, and upregulation of Sprr2b and Krt16 expression (Figure 5E–G, Figure S6E,F). Notably, the disruption of the epidermal barrier, once again, caused a clear fungal overgrowth (Figure 5H). As in the MC903 model, Malassezia overgrowth appeared to be independent of Tslp since the cytokine was strongly downregulated in response to the fungus in tape-stripped skin (Figure S6G). In addition, the observation that Il17a expression was not altered in the tape-stripped skin (Figure S6H) confirms our previous conclusion that fungal overgrowth in an AD-like environment is not primarily a consequence of dysregulated type 17 immunity. Together, our data show that epidermal barrier impairment, regardless of its genetic, chemical, or mechanical origin, strongly favored Malassezia growth and, thereby allowed the fungus to overcome existing antifungal defense mechanisms, and that this was accompanied by enhanced hyperplasia and epidermal thickness.
2.6 Transcriptional changes in lipid metabolism in AD-like skin support Malassezia adaptation in lipid acquisition and growth
Next, we sought to identify factors that benefit Malassezia growth in the AD-like skin. Because Malassezia relies on extracellular lipids to grow and AD is characterized by major abnormalities in lipid metabolism,63-65 we studied the relationship between the alterations in lipid metabolism and Malassezia overgrowth. The skin of flaky tail (ft/ft) mice, another widely used model of AD that is based on naturally acquired mutations in the Flg and Tmem79 genes, manifest an AD-like epidermal barrier defect.66, 67 We previously detailed the profound changes in the lipid metabolism in these mice, which are characterized by aberrant lamellar body (LB) cargo and secretory system, increased peroxisomal fatty acid oxidation, and accumulation of shorter chain fatty acid and ceramide species at the cost of very-long-chain epidermal lipids.54 The enriched lipids are within the spectrum of those preferentially utilized by Malassezia (C12–C20 fatty acids).68, 69 When exposing ft/ft mice to M. pachydermatis we observed enhanced fungal colonization in comparison to WT control mice (Figure 6A). The increased fungal burden coincided with an increase in the overall skin thickness and with epidermal hyperplasia after fungal association (Figure 6B,C) as seen before in other AD-like models (Figure 1B, Figure 6A,E).
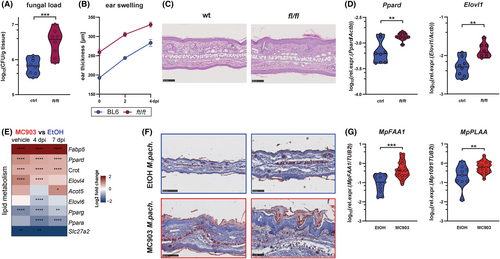
Dysregulation of cutaneous lipid metabolism and anaerobic glycolysis, a hallmark of the AD-like phenotype in ft/ft mice,54 persisted after Malassezia association (Figure 6D, Figure S7A). These lipid-related changes were conserved in the skin of MC903-treated and Nrf2-overexpressing mice (Figure 6E, Figure S7B,C), most prominently by transcriptional changes in the master regulator genes of lipid metabolism (Ppard, Pparg), as well as in fatty acid shuttling (Fabp5, Crot), uptake (Slc27a2), and synthesis/elongation (Elovl1, Elov4, Elov6) genes. These changes were independent of TSLP in the MC903 model (Figure S7B) and coincided with an increase in lipids at the site of Malassezia colonization in the stratum corneum including hair follicles (Figure 4C), as revealed by Oil Red staining (Figure 6F). Together, these data suggest that Malassezia takes advantage of the altered and more easily accessible metabolic niche in AD skin for its own thriving.
Given its lack of fatty acid synthase, Malassezia relies on the acquisition of lipids from the host by means of lipases, phospholipases, and sphingomyelinases.70 We detected increased expression of MpFAA1, a key fungal gene implicated in fatty acid uptake, activation and consequently in fungal growth,69, 70 in the MC903-treated skin (Figure 6G). Likewise, we found the fungal phospholipase A2 activating gene MpPLAA to be upregulated (Figure 6G). Phospholipases are involved in the acquisition of fatty acids from the host environment,71 and their production and activity is enhanced in M. pachydermatis isolates from dogs with dermatitis or otitis,72-74 emphasizing their relevance for Malassezia fitness in the atopic skin. Together, these findings suggest that by increasing its lipid uptake machinery Malassezia may benefit from the altered lipid environment in the barrier-impaired skin, which provides a growth advantage.
3 DISCUSSION
Malassezia is the predominant fungus of the skin microbiome. Although usually a commensal, it is also associated with a range of skin pathologies, which, besides AD, also include dandruff, pityriasis versicolor, and seborrheic dermatitis.6 Despite the high incidence and the resilience to therapy of many of these disorders, it remains largely unknown how Malassezia is involved in pathogenesis. Skin disorders have a high global prevalence. Although usually not lethal, they significantly limit the well-being of those affected and consequently are listed the 4th greatest non-fatal disabling condition, with fungal skin infections, AD, and pruritus ranking among the most frequent skin pathologies.75, 76
In this study we show that dysregulation of the epidermal barrier, as it is typical for AD, predisposes for excessive fungal growth, which in turn is associated with increased epidermal hyperplasia and inflammation. These findings were reproduced in four commonly used experimental models, each of which meets numerous key criteria of AD and/or ichthyosis. Together, our data from all different models used in this study strongly support the conclusion that structural and functional dysregulation of the skin barrier establishes an environment that is favorable for Malassezia colonization and to which Malassezia contextually adapts for enhanced nutrient assimilation.
Our findings are consistent with the situation in canine AD and hereditary ichthyosis, both conditions being commonly accompanied by Malassezia overgrowth in skin (Malassezia dermatitis) and the ear canal (otitis externa).77-80 In human AD, an increase in Malassezia colonization was evidenced by PCR in lesions of HND patients, a subtype of AD in which Malassezia-sensitization is closely correlated with disease severity.21, 81 Other studies assessing fungal colonization in human AD reported increased diversity in the skin mycobiome, which was interpreted as a reduction in Malassezia colonization.82 However, because microbiome studies commonly report relative abundance, reduction in the relative abundance of a given fungal species does not necessarily imply a reduction in absolute colonization loads despite other fungal taxa emerging. In contrast, a PCR based approach was employed for the detection of increased loads of Malassezia in the skin of HND patients.81
The changes in lipid organization of the stratum corneum, which in the ft/ft and MC903 models close recapitulate those in human and canine AD skin, are characterized by increased peroxisomal fatty acid oxidation, a reduction in ceramides and an accumulation of shorter chain fatty acids at the cost of very-long-chain fatty acids.54, 83-85 As ceramides bear antifungal properties, their decrease in AD skin was linked to enhanced Malassezia growth in AD skin.21 In turn, the fatty acids that are enriched in AD skin54 are within the spectrum of fatty acids that are preferentially utilized by Malassezia (C12-C20).68, 69 Our results further indicate that Malassezia adapts to the altered niche conditions in AD-like skin to its own advantage. M. pachydermatis responds to altered lipid availability in barrier dysregulated skin by increased expression of genes involved in enhanced lipid acquisition, such as MpFAA1 and MpPLAA. Long-chain fatty acyl-CoA synthetase 1 (FAA1) recognizes and facilitates uptake of C14–C16 fatty acids important for Malassezia growth.69, 70 PLAA in turn positively regulates cytosolic and calcium-independent phospholipase A2 activities. Phospholipases are a heterogeneous group of enzymes that hydrolyze ester linkages in phospholipids by cleaving specific ester bonds.86 Importantly, PLA2 activity was found increased in M. pachydermatis isolates from skin lesions of dogs presenting dermatitis and/or otitis, as reported by three independent studies.72-74 Phospholipases are considered virulence factors in many pathogenic fungi due to their potential to damage host cell membranes,86 which can further aggravate barrier disruption and cause inflammation by inducing host cell lysis and subsequent release of danger-associated molecular patterns. In addition, the hydrolysis of lipids by lipases may affect the acidic pH of the skin, as it is the case for bacterial lipases,87, 88 while enzyme activity itself is affected by pH.89, 90
While homeostatic immunity is central for controlling and restraining commensal organisms,3 changes in the immune status in barrier-impaired skin were insufficient to explain Malassezia overgrowth in the MC903 model. As such, type 2 cytokines were not directly responsible for the observed fungal overgrowth based on results obtained from IL-4cx injection experiments and from MC903-treated TSLPR-deficient mice. Accordingly, the ichthyosis phenotype and altered lipid profile in MC903-treated skin was independent of TSLP signaling. Besides type 2 immunity, immune dysregulation in the atopic skin in the MC903 model is hallmarked by reduced antifungal type 17 response. Although our results manifest a partial contribution of the compromised IL-17 production in the MC903-treated skin, the pronounced fungal overgrowth persisted irrespective of the IL-17 status of the host. As such, Malassezia can overcome antimicrobial defense and inflammation and effectively access favorable nutrients in the altered niche that foster enhanced colonization.
The use of murine models entails certain translational limitations. The MC903 model displays critical aspects of acute lesional AD with strong type 2 immune bias and cutaneous barrier impairment257, but cannot reflect all AD endotypes. Furthermore, the barrier defects observed in the MC903 model appeared to be largely independent of TSLPR signaling and type 2 immunity. In human subjects, type 2 cytokines can negatively affect barrier function, e.g. by downregulation of filaggrin expression or changes in the barrier lipid metabolism leading to barrier impairment and shorter chain lipid species.331,332 By using different models of AD (including flaky tail mice) as well as mimicking certain phenotypic aspects of the disease (Nrf2 overexpression for cellular stress and tape stripping for barrier disruption) we aimed at better encompassing the diversity of this disease to strengthen the observed effects. Additional parameters may impact Malassezia colonization in the AD-like skin such as a shift in the skin microbiome, which we have not assessed in our study. Dysbiosis may indirectly affect Malassezia colonization. Such effects may however be minimal in the MC903 model given the sterilizing effect of repeated application of EtOH, which serves as the solvent of MC903.
Irrespective of such putative additional factors, our results provide important novel insights into how the Malassezia-host interplay is impacted in AD-like skin and how it may add to the inflammatory pathology. Furthermore, it identifies an important role of MC903 in the modulation of skin fungal homeostasis, which should be considered for pharmacological use of calcipotriol and other Vitamin D analogs against skin carcinomas91, 92 and psoriasis (Dovonex®).42, 43 Therapeutic approaches to strengthen the epidermal barrier function can support the restoration of a homeostatic interaction with commensals and prevent their overt growth and aberrant expression of virulence traits. Maintaining stable commensalism is vital for host physiology given that fungal commensals influence many aspects of host health, barrier protection, host defense, and even social behavior.93
4 METHODS
4.1 Ethics statement
All mouse experiments in this study were conducted in strict accordance with the guidelines of the Swiss Animals Protection Law and were performed under the protocols approved by the Veterinary office of the Canton Zurich, Switzerland (license number 168/2018 and 142/2021). All efforts were made to minimize suffering and ensure the highest ethical and humane standards according to the 3R principles.94
4.2 Animals
WT C57Bl/6JRj and Balb/c mice were purchased from Janvier Elevage (France). Il17af−/−,95 Tcrd−/−,96 Rag2−/− Il2rg−/−97 (kindly obtained from the Swiss Immunological Mouse Repository), and MyD88−/− mice98 were bread at the Laboratory Animal Science Center of the University of Zurich (LASC). Il17af−/− mice were used with littermate and co-housed WT control mice (Il17afwt). MyD88−/− mice were used with heterozygous littermate controls. Flaky tail (ft/ft) mice66, 67 were bred at the Medical University of Innsbruck, Austria, and used for experiment with WT controls from the same facility. Tslpr−/− mice34 (kindly obtained from Daniela Finke) were bred at the Department of Biomedicine of the University of Basel and used for experiment with WT controls from the same facility. K5cre-CMVcaNrf2 and caNrf2 control mice60 (kindly obtained from Sabine Werner) were bred at the ETH Zurich Phenomics Center. All mice were on the C57BL/6 background and kept under specific pathogen-free conditions. All experiments were conducted at the Laboratory Animal Science Center of the University of Zurich. Female as well as male mice were used at 8–15 weeks of age in sex- and age-matched groups. Groups of vehicle-treated and infected animals were kept in separate cages to avoid cross-contamination.
4.3 Fungal strains
All Malassezia strains used in this study were used previously in the Leibundgut lab.14 M. pachydermatis strain ATCC 14522 (CBS 1879)99 was originally purchased from ATCC. M. sympodialis strain ATCC 42132100 and M. furfur strain JPLK23 (CBS 14141)99 were originally obtained from Joseph Heitman (Duke University Medical Center, Durham, NC). All strains were grown in liquid modified Dixon (mDixon) medium (Malt Extract (Sigma-Aldrich), desiccated Ox-bile (Sigma-Aldrich), Tween-40 (Sigma-Aldrich), Peptone (Oxoid), Glycerol (Honeywell), Oleic Acid (Sigma-Aldrich)) at 30°C and 180 rpm for 2–3 days. Heat-killed Malassezia was prepared by twice washing Malassezia grown from liquid culture in PBS and exposing to 85°C for 45 min at a concentration of OD5A600/ml.
4.4 MC903-induced model of AD
Induction of an AD-like skin phenotype by MC903 was based on Moosbrugger-Martinz et al.29 In short, both ears of mice were treated with MC903 (calcipotriol hydrate; Sigma) in ethanol or with pure ethanol as a control (Sigma) on the dorsal and ventral side of both ears. The dose of MC903 was 1.125 nmol in 25 μL ethanol per ear. The treatment was done daily for 5 days and again 4 days after a resting period of 2 days. The treatment was continued after infection on the ventral ear side only on day 2 (experiments with endpoint at 4 dpi) or daily from day 2 to 5 (experiments with endpoint at 7 dpi). For experiments with Balb/c mice, left ears were treated with EtOH while the right ear was treated with MC903. Ear thickness was continuously monitored using the Oditest S0247 0–5 mm measurement device (Kroeplin).
4.5 Systemic treatment of mice with IL-4cx
IL-4cx, composed of 0.5 μg mouse IL-4 (PeproTech) complexed with 5 μg anti-IL-4 mAb (clone 11B11; Bio X cell) was injected i.p. daily from day -2 until day 0 as described previously.101 Mice received further IL-4cx injections on days 2, 4, and 6 post-infection with Malassezia.
4.6 Tape stripping of mouse ear skin
Tape stripping was performed by repeated application and removal of adhesive tape (Transpore™ Hypoallergenic, 3M; 10 times per ear). Ear thickness was measured using the Oditest S0247 0–5 mm measurement device (Kroeplin).
4.7 Epicutaneous association of mouse ear skin with Malassezia
Epicutaneous infection of the mouse ear skin was performed as described previously.14, 31 Malassezia strains grown in mDixon medium were washed in PBS and suspended in native olive oil at a density of 10 ODA600/ml. 100 μL suspension (corresponding to 1 ODA600 or 5 × 106 CFU/ml) of yeast cells was applied topically onto the dorsal ear skin while mice were anesthetized. For determining the fungal loads in the skin, tissue was transferred in water supplemented with 0.05% Nonidet P40 (AxonLab), homogenized with a TissueLyzer (Qiagen) for 6 min at 25 Hz, plated on mDixon agar, and incubated at 30°C for 3 to 4 days.
4.8 Histology
Mouse tissue was fixed in 4% PBS-buffered paraformaldehyde overnight and embedded in paraffin. Sagittal sections (2-8 μm) were stained either with hematoxylin and eosin (H&E) or with Periodic-acidic Schiff (PAS) reagent and counterstained with Hematoxylin and mounted with Pertex (Biosystem, Switzerland) according to standard protocols. Oil red staining was performed and counterstained with Hemalum solution and mounted on aqueous mounting medium according to standard protocols. All images were acquired with a digital slide scanner (NanoZoomer 2.0-HT, Hamamatsu) and analyzed with NDP view2. Epidermal thickness was determined by blinded measurements on two histological sections per mouse with eight measurements each using ImageJ software (version 1.52a).
4.9 RNA isolation and RT-qPCR
Isolation of total RNA from murine ear skin was performed using TRI reagent (Sigma-Aldrich) according to the manufacturer's protocol with the modification that the tissue was homogenized using a TissueLyzer (Qiagen) for 6 min at 25 Hz before extraction of the nucleic acids with chloroform. For isolation of fungal RNA, an additional step of homogenization with glass beads (0.5 mm, Sigma) was included twice for 2 min at 30 Hz interrupted by 30 s cooling periods on ice. cDNA was generated by RevertAid reverse transcriptase (ThermoFisher) and random nonamers. Quantitative PCR was performed using SYBR green (Roche) and a QuantStudio 7 Flex (Life Technologies) or a 7500 Real-Time PCR System (Applied Biosystems) instrument. The primers used for qPCR are listed in Table S1. All RT-qPCR assays were performed in duplicate, and the relative expression (rel. expr.) of each gene was determined after normalization to mouse Actb or M. pachydermatis TUB2 housekeeping genes. Fungal cDNA data was excluded when expression of the housekeeping gene was above a Ct value of 40 or when unspecific amplification occurred.
4.10 Bulk RNA-seqencing
RNA sequencing was performed by the Functional Genomics Center Zürich. RNA was extracted using the Direct-zol RNA MiniPrep Plus Kit (Qiagen) following manufacturer's protocol. Extracted RNA was prepared for sequencing using the Illumina TruSeq RNA assay following manufacturer's protocol. Sequencing was performed on the Illumina NovaSeq 6000 using the S1 Reagent Kit v1.5 (100 cycles) as per manufacturer's protocol. Demultiplexing was performed using the Illumina bcl2fastq Conversion Software. Individual library size ranged from 20.6 million to 28.5 million reads.
4.11 RNA-Seq data analysis
RNA sequencing analysis was performed using the SUSHI framework,102 which encompassed the following steps: Read quality was inspected using FastQC, and sequencing adaptors removed using fastp; Alignment of the RNA-Seq reads using the STAR aligner103 and with the GENCODE mouse genome build GRCm39 (Release M26) as the reference104; the counting of gene-level expression values using the “featureCounts” function of the R package Rsubread105; differential expression using the generalized linear model as implemented by the DESeq2 Bioconductor R package,106 and; Gene Ontology (GO) term pathway analysis using the hypergeometric over-representation test via the “enrichGO” function of the clusterProfiler Bioconductor R package.107 Plots were created using the SUSHI framework including the exploreDEG Interactive Shiny App (https://doi.org/10.5281/zenodo.8167438). All R functions were executed on R version 4.1 (R Core Team, 2020) and Bioconductor version 3.14.
4.12 Isolation of murine skin and lymph node cells
For digestion of total ear skin, mice ears were removed, cut into small pieces, and transferred into Hank's medium (Ca2+- and Mg2+-free, Life Technology), supplemented with Liberase TM (0.15 mg/mL, Roche) and Dnase I (0.12 mg/mL, Sigma-Aldrich) and incubated for 1 h at 37°C. Ear-draining lymph nodes (dLN) were digested with DNAse I (2.4 mg/mL Sigma-Aldrich) and Collagenase I (2.4 mg/mL, Roche) in PBS for 15 min at 37°C. Both cell suspensions were filtered through a 70 μm cell strainer (Falcon) and rinsed with PBS supplemented with 5 mM EDTA (Life Technologies), 1% fetal calf serum and 0.02% NaN3. Single cell suspensions were used for re-stimulation or stained directly for flow cytometry.
4.13 Ex vivo T cell stimulation
For in vitro re-stimulation of skin T cells, skin cell suspensions were incubated in a U-bottom 96-well plate (1/4 ear per well) with cRPMI medium (RPMI supplemented with 10% fetal calf serum, HEPES, sodium pyruvate, non-essential amino acids, β-mercaptoethanol, Penicillin and Streptomycin) with phorbol 12-myristate 13-acetate (PMA, 50 ng/mL, Sigma-Aldrich) and ionomycin (500 ng/mL, Sigma-Aldrich) for 5 h at 37°C, 5% CO2 in the presence of Brefeldin A (10 μg/mL). For in vitro re-stimulation of lymph node T cells, 106 lymph node cells per well of a flat bottom 96-well plate were exposed to PMA (50 ng/mL, Sigma-Aldrich) and ionomycin (500 ng/mL) or co-cultured with 1 × 105 DC1940 cells108 that were pulsed with 2.5 × 105 heat-killed fungal cells. Brefeldin A (10 mg/mL, Sigma-Aldrich) was added for the last 4 hours of incubation at 37°C, 5% CO2.
4.14 Flow cytometry
Single cell suspensions were stained with antibodies as listed in the Table S1. LIVE/DEAD Near IR stain (Life Technologies) or Zombie Aqua fixable viability staining (Biolegend) was used for exclusion of dead cells. After surface staining, cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences) for subsequent intracellular staining in Perm/Wash buffer (BD Bioscience). All staining steps were carried out on ice. Data were acquired on a Spectral Analyzer SP6800 (Sony), CytoFLEX S (Beckman Coulter) or 5L Cytek Aurora (Cytek). All fcs files were analyzed with FlowJo software V10 (FlowJo LLC). Gating of the flow cytometric data was performed according to the guidelines for the use of flow cytometry and cell sorting in immunological studies,109 including pre-gating on viable and single cells for analysis. Absolute cell numbers were calculated based on a defined number of counting beads (BD Bioscience, Calibrite Beads) that were added to the samples before flow cytometric acquisition.
4.15 ELISA
Blood was collected by post-mortem cardiac puncture and transferred to blood collection tubes (BD Microtainer®, BD) for serum isolation. Detection of IgE from mouse serum was performed by ELISA using ELISA MAX™ Standard Set Mouse IgE (Biolegend) according to the manufacturer's instructions.
4.16 Quantification and statistical analysis
Statistical significance was determined by unpaired Student's t-test with Welch's correction and one- or two-way ANOVA with Dunnet's or Tukey's multiple comparison test, as appropriate, using GraphPad Prism software. Data displayed on a logarithmic scale were log-transformed before statistical analysis. Significance is indicated as follows: *p < .05; **p < .01; ***p < .001; ****p < .0001.
AUTHOR CONTRIBUTIONS
F.R. and S.L.-L. designed the study and wrote the original draft of the manuscript. F.R. performed the experiments and analyzed the data. P.Z. developed the multiparameter flow cytometry panel in Figure S2 and analyzed the data together with F.R. S.L.-L. oversaw all study design and data analysis and acquired funding. S.D. provided flaky tail mice and important intellectual input. B.B. provided intellectual input. All authors discussed the results, and reviewed and edited the manuscript.
ACKNOWLEDGMENTS
The authors would like to thank Daniela Finke (University of Basel, Department of Biomedicine, Basel, Switzerland) for Tslpr−/− mice; Sabine Werner and Michael Koch (Institute for Molecular Health Sciences, ETH Zurich, Switzerland) for K5cre-CMVcaNrf2 mice; Onur Boyman for anti-IL-4 antibody; the staff of the Laboratory Animal Service Center of University of Zurich for animal husbandry; staff of the Laboratory for Animal Model Pathology of University of Zurich for histology; Peter Leary from the Functional Genomics Center Zürich for bioinformatics support; members of the LeibundGut-lab and of SKINTEGRITY.CH for helpful advice and discussions. This work was supported by the Swiss National Science Foundation (grant # 310030_189255, to S.L.-L.) and by a Candoc grant from the University of Zürich (grant #5843, to F.R.). Open access funding provided by Universitat Zurich.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interests to declare.
Open Research
DATA AVAILABILITY STATEMENT
RNAseq data have been deposited on the NCBI GEO repository, accession number GSE253214. All other raw data linked to this study have been made publicly available on Zenodo (https://doi.org/10.5281/zenodo.10667318).