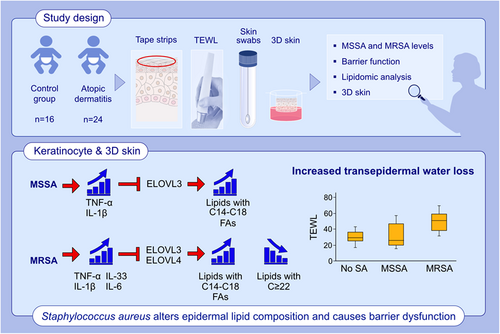
Staphylococcus aureus causes aberrant epidermal lipid composition and skin barrier dysfunction
Jihyun Kim and Byung Eui Kim contributed as equal first authors to this work.
Abstract
Background
Staphylococcus (S) aureus colonization is known to cause skin barrier disruption in atopic dermatitis (AD) patients. However, it has not been studied how S. aureus induces aberrant epidermal lipid composition and skin barrier dysfunction.
Methods
Skin tape strips (STS) and swabs were obtained from 24 children with AD (6.0 ± 4.4 years) and 16 healthy children (7.0 ± 4.5 years). Lipidomic analysis of STS samples was performed by mass spectrometry. Skin levels of methicillin-sensitive and methicillin-resistant S. aureus (MSSA and MRSA) were evaluated. The effects of MSSA and MRSA were evaluated in primary human keratinocytes (HEKs) and organotypic skin cultures.
Results
AD and organotypic skin colonized with MRSA significantly increased the proportion of lipid species with nonhydroxy fatty acid sphingosine ceramide with palmitic acid ([N-16:0 NS-CER], sphingomyelins [16:0–18:0 SM]), and lysophosphatidylcholines [16:0–18:0 LPC], but significantly reduced the proportion of corresponding very long-chain fatty acids (VLCFAs) species (C22–28) compared to the skin without S. aureus colonization. Significantly increased transepidermal water loss (TEWL) was found in MRSA-colonized AD skin. S. aureus indirectly through interleukin (IL)-1β, tumor necrosis factor (TNF)-α, IL-6, and IL-33 inhibited expression of fatty acid elongase enzymes (ELOVL3 and ELOVL4) in HEKs. ELOVL inhibition was more pronounced by MRSA and resulted in TEWL increase in organotypic skin.
Conclusion
Aberrant skin lipid profiles and barrier dysfunction are associated with S. aureus colonization in AD patients. These effects are attributed to the inhibition of ELOVLs by S. aureus-induced IL-1β, TNF-α, IL-6, and IL-33 seen in keratinocyte models and are more prominent in MRSA than MSSA.
Graphical Abstract
MRSA induces a broad range of cytokines, while MSSA induces IL-1β and TNF-α. Staphylococcus aureus-induced IL-1β, TNF-α, and IL-6 inhibit ELOVL3, resulting in accumulation of lipids with C14-C18 FAs, while MRSA-induced IL-33 inhibits both ELOVL3 and ELOVL4, concomitantly increasing lipids with C14-C18 FAs and decreasing lipids with FAs ≥ 22. S. aureus-mediated aberrant lipid profiles cause increased TEWL and skin barrier dysfunction.Abbreviations: 3D skin, organotypic skin; ELOVL, fatty acid elongase; FA, fatty acid; IL, interleukin; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive Staphylococcus aureus; SA, Staphylococcus aureus; TEWL, transepidermal water loss; TNF, tissue necrosis factor
Abbreviations
-
- AD
-
- atopic dermatitis
-
- BSA
-
- bovine serum albumin
-
- CERS
-
- ceramide synthase
-
- CER
-
- ceramide
-
- CYP4F22
-
- cytochrome P450 family 4 subfamily F polypeptide 22
-
- DEGS2
-
- delta 4-desaturase, sphingolipid 2
-
- ELOVL
-
- elongation of very long-chain fatty acids protein
-
- FA
-
- fatty acid
-
- HEKs
-
- human primary epidermal keratinocytes
-
- IL
-
- interleukin
-
- LDH
-
- lactate dehydrogenase
-
- LPS
-
- lipopolysaccharide
-
- LC-ESI-MS/MS
-
- liquid chromatography electrospray ionization tandem mass spectrometry
-
- LCFA
-
- long-chain fatty acid
-
- LPC
-
- lysophosphatidylcholine
-
- MRSA
-
- methicillin-resistant Staphylococcus aureus
-
- MSSA
-
- methicillin-sensitive Staphylococcus aureus
-
- MFI
-
- mean fluorescence intensity
-
- NS-CER
-
- nonhydroxy fatty acid sphingosine ceramides
-
- PVL
-
- Panton-Valentine leucocidin
-
- PSM
-
- phenol-soluble modulin
-
- PCR
-
- polymerase chain reaction
-
- HEK
-
- primary human epidermal keratinocyte
-
- RT-PCR
-
- reverse transcription-polymerase chain reaction
-
- SCORAD
-
- SCORing Atopic Dermatitis
-
- STS
-
- skin tape strip
-
- SM
-
- sphingomyelin
-
- S
-
- Staphylococcus
-
- SC
-
- stratum corneum
-
- TEWL
-
- transepidermal water loss
-
- TNF
-
- tumor necrosis factor
-
- UGCG
-
- UDP-glucose ceramide glucosyltransferase
-
- VLCFA
-
- very long-chain fatty acid
1 INTRODUCTION
The stratum corneum (SC), the outermost layer of the epidermis, provides a physical and functional barrier to prevent invasion of allergens, pathogens, and chemicals into the human body.1-3 Dead corneocytes and intercellular lipids are major components of the SC and form a strong hydrophobic barrier, which prevents excessive water loss through the skin.2, 4 The intercellular skin lipids consist of ceramides (CERs), cholesterol, and fatty acids (FAs), and CERs are essential lipid components in the SC.2, 4, 5
Atopic dermatitis (AD) is characterized by epidermal barrier defects and recurrent bacterial infections such as Staphylococcus (S) aureus.4, 6 Studies have determined that up to 60%–100% of AD patients are skin colonized by S. aureus as compared to 5%–30% of healthy controls.7, 8 Moreover, it is estimated that up to 10%–30% of S. aureus isolates from AD skin cultures are methicillin-resistant S. aureus (MRSA), and the prevalence of MRSA infection in the skin is increasing.8-10 S. aureus colonization in the skin exacerbates AD and may contribute to microbial dysbiosis, allergen sensitization, T helper type (Th) 2/Th17 polarization, development of the atopic march, and food allergy in patients with AD.8, 9
New insights into the pathophysiology of AD have highlighted the pivotal roles of abnormalities in the epidermal lipid layer and cutaneous microbial dysbiosis.3, 5, 8, 11 AD skin often demonstrates a loss in bacterial diversity and dysbiosis due to increased S. aureus during AD flares.3, 12, 13 Altered epidermal lipid composition and increased transepidermal water loss (TEWL) were reported in AD skin.11, 14, 15
Although it has been suggested that S. aureus colonization in AD skin is associated with changes in skin barrier lipid compositions,16 the specific mechanisms involved in S. aureus-directed changes in epidermal lipid profiles have not been studied. Thus, we have hypothesized that S. aureus causes aberrant lipid profiles and skin barrier dysfunction. In the present study, we evaluated skin lipid profiles in pediatric AD patients according to the types of S. aureus colonization and assessed whether S. aureus induces abnormal lipid profiles and subsequent skin barrier dysfunction.
2 METHODS
2.1 Study subjects
Skin tape strip (STS) samples were obtained from 24 children with AD (6.0 ± 4.4 years) and 16 healthy subjects (7.0 ± 4.5 years) (p = .217). The groups were well matched for gender distribution, with 59.3% of males in the AD group and 50% of males in the healthy subjects group (p = .54). Subjects in the healthy control group had no personal or family history of atopy or skin diseases. AD patients recruited for this study did not use topical corticosteroids, topical calcineurin inhibitors, or topical or oral antibiotics for 1 week prior to enrollment. Patients were not treated with systemic immunosuppressive medications for more than 1 month prior to enrollment into the present study. The diagnosis of AD was based on the criteria defined by Hanifin and Rajka.17 AD severity was assessed by allergists using the SCORing Atopic Dermatitis (SCORAD), with the score range from 0 to 103.18 TEWL and skin pH were measured on non-lesional skin from the volar surface of the forearm and lesional skin. All TEWL measurements were done by Tewameter TM300 (Courage & Khazaka, Köln, Germany). In the test room, the relative humidity was kept at 40–50% and temperature was kept at 22–24°C as previously done.19 This study was approved by the Institutional Review Board at Samsung Medical Center, Pusan National University Hospital, and Inje University-Sanggye Paik Hospital (IRB No. SMC-2018-03-041, H-1808-021-070, and SGPAIK 2018-06-005-001, respectively). Written informed consent was obtained from all parents and/or patients prior to participation in this study.
2.2 Collection of skin tape strip samples
We obtained three different types of skin samples for STS and bacterial swabs: (1) lesional skin from 24 AD patients, (2) non-lesional volar forearm skin from 24 AD patients, and (3) normal skin from 16 healthy control subjects. A total of four consecutive D-Squame® tape discs (22 mm diameter; CuDerm) were applied to the skin as described before.20 Bacterial DNA samples from skin swabs were prepared for polymerase chain reaction (PCR) as described previously.21
2.3 Tape strip processing for lipid extraction and protein estimation
Tape strip processing was performed according to the previously published protocol.22 Lipids were dissolved in 0.2 ml methanol for liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) analyses.
2.4 Lipid and protein analyses
CERs, lysophosphatidylcholines (LPCs), and sphingomyelins (SMs) from human skin and organotypic 3D skin were identified and quantified using the targeted LC-ESI-MS/MS approach on a Sciex 6500QTRAP mass spectrometer coupled with a Shimadzu Nexera X2 UHPLC system as described previously.11
2.5 Primary human keratinocyte culture
Human primary epidermal keratinocytes (HEKs) derived from neonatal foreskin were grown in serum-free EpiLife cell culture medium (Life Technologies, Grand Island, NY) containing 0.06 mmol/L calcium chloride, 1% human keratinocyte growth supplement S7 (Life Technologies), and 1% of gentamicin/amphotericin.
To investigate the effects of the Staphylococci on the gene expressions of FA elongases (ELOVLs), CER synthase (CERS)2, CERS3, cytochrome P450 family 4 subfamily F polypeptide 22 (CYP4F22), delta 4-desaturase, sphingolipid 2 (DEGS2), and UDP-glucose ceramide glucosyltransferase (UGCG), HEKs were seeded in a 24-well plate with 2 × 105 cells per well and differentiated in the presence of 1.3 mmol/L of CaCl2 for 3 days. Then, cells were stimulated with three strains of live S. epidermidis (ATCC®-12228™, ATCC®-49461™, and ATCC®-700296™, Manassas, VA, 0.001 or 0.01 CFU/well), three strains of methicillin-sensitive S. aureus (MSSA) (ATCC®-29213™, ATCC®-49230™, and SMC1801-45, Asian Bacterial Bank, Seoul Korea, 0.001 or 0.01 CFU/well), or three strains of MRSA (ATCC®-BAA-1556™, ATCC-700699™, and SMC1803-07, Asian Bacterial Bank, 0.001 or 0.01 CFU/well) for an additional 24 h, prior to RNA collection. Cell culture media were also harvested and stored at −80°C for lactate dehydrogenase (LDH) assays.
To examine Staphylococci-induced cytokine expression, HEKs were differentiated for 3 days, and then, cells were stimulated with three strains of S. epidermidis (same as above, 0.01 CFU/well), MSSA (same as above, 0.01 CFU/well), or MRSA (same as above, 0.01 CFU/well) for 8 or 20 h. Cell culture media were harvested and stored at −80°C for ELISA assays.
To investigate whether keratinocyte-derived cytokines inhibit ELOVL3 and ELOVL4, HEKs were Ca2+ differentiated and stimulated with various concentrations of S. aureus-induced cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-36γ, IL-6, and IL-33 for 24 h. In addition, HEKs were differentiated and preincubated with isotype control antibodies or neutralizing antibodies against TNF-α (0.1 μg/ml; R&D Systems), IL-1β (10 ng/ml; R&D Systems), IL-36γ (1.0 μg/ml; R&D Systems), IL-6 (0.1 μg/ml; R&D Systems), and IL-33 (1.0 μg/ml; R&D Systems) separately or in combination for 3 h, followed by stimulation with MSSA (0.01 CFU/cell) or MRSA (0.01 CFU/cell) for an additional 20 h.
2.6 Real-time RT-PCR
RNeasy Mini Kits (Qiagen) were used for RNA extraction from cultured HEKs. RNA was reverse-transcribed into cDNA using the SuperScript VILO MasterMix (Invitrogen). Real-time reverse-transcribed polymerase chain reaction (RT-PCR) was performed using an ABI Prism 7300 sequence detector (Applied Biosystems). Primers and probes were purchased from Applied Biosystems. Amplification reactions were performed in MicroAmp optical plates (Applied Biosystems) as previously described.23 Relative expression levels were calculated by the relative standard curve method as outlined in the manufacturer's technical bulletin.
2.7 Organotypic skin model
A mixture of fibroblasts (7.5 × 105/culture insert; CCL-92™, ATCC) and rat tail collagen type I (10 mg/ 2 ml/culture insert; Corning Life Sciences) were plated on tissue culture inserts (MilliporeSigma) to prepare dermal equivalents. HEKs (2 × 106/culture insert) were then plated on top of the dermal equivalent and cultured for 12 days. On Day 13, the organotypic 3D skins were stimulated with media and live S. aureus (0.01 CFU/cell) for an additional 20 h. The epidermal part of the organotypic skin was isolated and stored at −80°C for lipidomic analysis or submerged in 4% paraformaldehyde to fix the tissue for immunostaining.
2.8 TEWL measurement
Transepidermal water loss (TEWL) in human skin was measured as previously described.19 The relative humidity was kept at 40%–50%, and the temperature was kept at 22–24°C in this room. TEWL in organotypic skin was measured using a device from GPower Inc. (Seoul, Korea) as previously described.22 To measure TEWL in organotypic skin, culture media was removed from the culture chamber and insert. Then, the organotypic skin sat for 10 minutes to equilibrate. TEWL was measured 3 times from each organotypic skin cultures, and the average values were used.
2.9 Preparation of bacteria
Fifty μL of frozen bacterial stock was added into 3 ml of tryptic soy broth (Millipore Sigma) and incubated at 37°C overnight on a rotary shaker. One hour prior to use, 3 ml of fresh tryptic soy broth media (Millipore Sigma) was added to the culture and then returned to incubation. One ml of bacterial culture was pelleted by centrifugation at 2850g-force for 5 min in an Eppendorf 5424 centrifuge, and then, CFUs/ml were determined by optical density at 595 nm using a spectrophotometer Scientific (Cole-Parmer®). Another 1 ml of bacterial culture was pelleted by centrifugation, resuspended in sterile PBS (1 × 107 cfu/ml) and then diluted to the desired concentration for use.
2.10 Multiplex real-time RT-PCR for Staphylococcus genes
The TaqMan probe-based multiplex real-time RT-PCR assay was performed by the method described in the previous reference.21 The multiplex real-time RT-PCR primers and TaqMan probes were used to amplify the Staphylococcus spp. (16S rRNA), S. aureus (nuc), and MRSA (mecA). Multiplex real-time RT-PCR was performed following conditions: 95°C for 3 min and 45 cycles of 95°C for 20 s and 60°C for 40 s in a single real-time RT-PCR assay.
2.11 Measurement of protein levels of TNF-α and IL-1β
Protein expression levels of TNF-α and IL-1β in cell culture supernatant were measured using an ELISA kit (R&D Systems) according to the manufacturer's instructions.
2.12 LDH assay
Culture media from HEKs culture and 3D skin experiments were collected and evaluated to examine cell toxicity of Staphylococci. LDH release was determined using the CytoTox-One Homogeneous Membrane Integrity Assay (Promega) according to the manufacturer's instructions.
2.13 Immunofluorescent staining
Organotypic skin sections were blocked with Super Block (Scyteck Laboratories). Slides were then stained with antibodies against ELOVL3 (Abcam; cat# ab253016, 1:500 dilution) and ELOVL4 (Abcam; cat# ab224608, 1:200 dilution). The slides were visualized with the fluorescent microscopy (Leica), and the levels of mean fluorescent intensity (MFI) were measured with Slidebook 6.0 (Intelligent Imaging Innovations).
2.14 Statistical analysis
Data were analyzed using SPSS for Windows (version 27.0; SPSS) and Graphpad Prism (version 9; Graphpad Software). Categorical data are presented as numbers and percentages. Continuous data are presented as median and interquartile ranges. Categorical variables between groups were compared using the chi-square test and Fisher's exact test. In comparisons of the non-lesional and lesional skins with the control group, data were analyzed by the Kruskal–Wallis test with the post hoc test. Statistical differences between the two groups were determined using a Mann–Whitney U-test. A p value < .05 was considered to be significant.
For details about lipidomics, organotypic skin model, LDH assay, TEWL measurement, RT-PCR, immunofluorescent staining, and bacterial DNA preparation for PCR from skin swabs, refer to the supplemental method file.
3 RESULTS
3.1 Increased TEWL and altered lipid profiles in the skin of AD children colonized with MRSA
We have evaluated SCORAD, TEWL, and epidermal lipid profiles of pediatric AD patients and normal healthy controls. We also performed PCRs to evaluate S. aureus colonization using skin swab samples, and subjects were then subgrouped based on S. aureus methicillin sensitivity into MSSA and MRSA groups (Table S1). Importantly, 43.4% of S. aureus-positive swabs from AD lesional skin were found to be MRSA positive, while 8.3% swabs from AD non-lesional skin and 6.3% swabs from normal healthy skin were MRSA positive (all p < .001) (Figure S1A).
AD severity (SCORAD score) was significantly (p < .01) higher in AD patients colonized with MRSA (median 31.6, IQR 28.8–33.7) than in the no S. aureus group (median 21.9, IQR 21.2–22.5), while no difference in SCORAD score was noted between the MSSA group (median 23.7, IQR 23.6–25.0) and the no S. aureus group (Figure S1B) in AD patients. Lesional skin TEWL of MRSA group was significantly higher as compared to no S. aureus group (p < .01) and MSSA group (p <.01) (Figure 1A). Additionally, non-lesional skin TEWL of the MRSA group was significantly (p < .05) higher compared to the no S. aureus group (Figure 1A). These data suggest that AD patients colonized with MRSA have more severe AD symptoms and skin barrier disruption than AD patients colonized with MSSA.
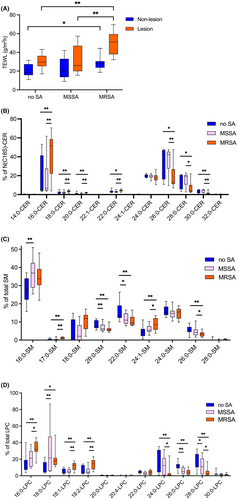
We have examined whether these changes in MRSA-colonized skin can be further corroborated by changes in epidermal barrier lipid profiles. Lipid molecular species with palmitic and stearic fatty acids (C16–C18) were globally increased in the skin samples from patients colonized with S. aureus (Figure 1). Nonhydroxy fatty acid C18-sphingosine ceramides (NS-CERs) N-acylated with C16–C22 FAs (Figure 1B), SM N-acylated with C16 & C17 FAs (Figure 1C), and LPCs with C16–C18 FAs (Figure 1D) were significantly increased in the skin samples colonized with MRSA compared to skin colonized with MSSA or without S. aureus colonization (p < .05 for all corresponding comparisons). Additionally, SM N-acylated with palmitic acid (C16) was significantly (p < .01) increased in the skin colonized with MSSA compared to that of skin without S. aureus colonization (Figure 1C). In contrast, very long chain fatty acid (VLCFAs) containing NS-CERs (FA carbon chain length C26–C30, Figure 1B), SMs (C26, Figure 1C), and LPCs (C24 and C28, Figure 1D) were decreased in the skin samples colonized with MRSA compared to skin colonized with MSSA or without S. aureus colonization. Additionally, SMs (with C20 and C22 FAs) and LPCs (with C26 and C28 FAs) were significantly (p < .05) decreased in the skin colonized with MSSA compared to skin without S. aureus colonization (Figure 1C,D). SM with C24:1 FA was significantly increased in the skin samples colonized with MRSA compared to those colonized with MSSA or without S. aureus colonization (Figure 1C). SM (with C16 FA) and LPCs (with C18 FA) were significantly (p < .01 for all) increased in the skin colonized with MSSA compared to skin without S. aureus colonization (Figure 1C,D). These data demonstrate that S. aureus colonization of skin is associated with aberrant lipid profiles, with more pronounced changes toward lipids with shorter-chain FAs (C16-C18) observed in the MRSA-colonized skin.
3.2 S. aureus increases TEWL and causes abnormal lipid profiles in organotypic skin cultures
We have modeled the effects of MSSA and MRSA exposure on skin barrier function and lipid profiles using organotypic skin cultures.
TEWL was not affected in organotypic skin cultures exposed to MSSA but was significantly increased in organotypic skin cultures after MRSA inoculation (p < .05 as compared to cultures exposed to MSSA; p < .001, as compared to cultures grown in media alone) (Figure 2A). These data suggest that MRSA, not MSSA, may cause skin barrier dysfunction.
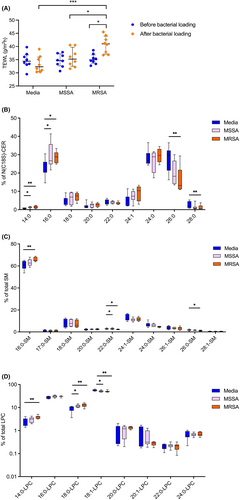
An increase in the amounts of C14 and C16 FAs in C18-sphingosine containing-CERs (Figure 2B) and an increased in LPC with stearic acid (18:0) (Figure 2D) were found in organotypic cultures stimulated by three different MSSA strains. In addition, some minor changes in N-22:0 SM and 18:1-LPC were observed in MRSA-stimulated cultures (Figure 2C,D). More pronounced changes were observed in organotypic cultures stimulated with three different MRSA strains. Namely, C14 and C16 FAs of N(С18S)-CERs (Figure 2B, p < .01, p < .05, respectively), N-16:0-SM (Figure 2C, p < .01), and LPCs with 14:0- and 18:0-fatty acids (Figure 2D, p < .01) were significantly increased in organotypic skin cultures stimulated with MRSA compared to the skin cultures stimulated with media alone. In contrast, the amounts of VLCFAs in N(C18S)-CERs (Figure 2B, C24 and C26) and LPCs with C18:1 FA (Figure 2D) were significantly decreased in organotypic skin cultures stimulated with MRSA compared to those stimulated with media alone. These data strongly support that MRSA exposure alters production of epidermal lipids with shorter chain fatty acids (C16–C18) and VLCFAs (C24–C28), resulting in accumulation of lipid species with C16–C18 FAs, which is likely detrimental to the skin barrier. Exposure to MSSA strains resulted in an increase in C16–C18 FA lipid species only, with no inhibition of C24–C28 FA lipid species production.
3.3 S. aureus modulates expression of fatty acid elongases
In mammalian cells, FA elongation proceeds through repetition of the FA elongation cycle whereby two carbons are added to the carboxyl end in each cycle. This reaction is catalyzed by FA ELOVLs; they constitute the ELOVL family of proteins, which includes seven isozymes (ELOVL1-7).24-26 We investigated whether Staphylococci exposure modulates ELOVL production in keratinocytes. In these experiments, we have compared ELOVL expression in keratinocyte cultures that were stimulated with 0.001 and 0.01 CFU of S. aureus (three different strains of MSSA and MRSA were tested, respectively) as compared to S. epidermidis, a commensal strain of Staphylococci which often colonizes human skin (three different S. epidermidis strains were used).
As depicted in Figure 3A, gene expression of ELOVL5 was significantly (p < .05) increased in HEKs stimulated with S. epidermidis, but other ELOVLs were not affected by S. epidermidis. In contrast, MSSA significantly (p < .01) inhibited ELOVL3 (Figure 3B) expression by keratinocytes. Furthermore, MRSA significantly inhibited both ELOVL3 (p < .001) and ELOVL4 (p < .05) (Figure 3C). Strain-dependent differences in MSSA and MRSA inhibition of ELOVLs were noted (Figure S2A,B).
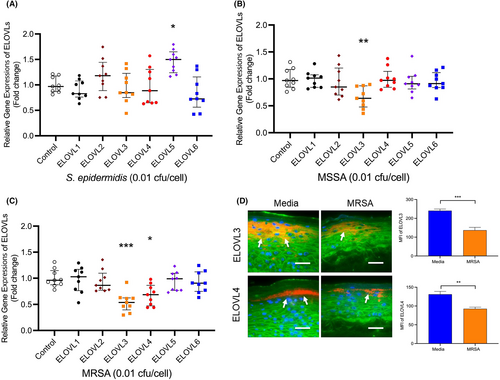
Staining intensities of ELOVL3 (p < 001) and ELOVL4 (p < .01) were significantly decreased in organotypic skin cultures stimulated with MRSA compared to skin cultures stimulated with media alone (Figure 3D). However, 0.001 cfu/cell of all Staphylococci (S. epidermidis, MSSA, MRSA) did not affect gene expression of ELOVLs (Figure S3A–C). Other enzymes26 that are involved in the synthesis of CERs and VLCFAs (including CERS2, CERS3, CYP4F22, DEGS2, and UGCG) were not affected by MRSA (Figure S3D).
Additionally, UV light-killed S. aureus and Escherichia coli lipopolysaccharide (LPS) did not inhibit ELOVL3 and ELOVL4 (Figure S4). Moreover, the effects of toxins produced by MSSA and MRSA including Panton-Valentine leucocidin (PVL) and phenol-soluble modulin alpha3 (PSMα3) were examined. PVL did not inhibit ELOVL3 and ELOVL4, but 10 μg/mlof PSMα3 significantly (p < .01) inhibited gene expression of ELOVL3 only (Figure S5).
Of note, keratinocyte cultures exposed to Staphylococci remained viable with minimal cytotoxicity observed in both organotypic skin cultures (Figure S6A) and HEKs (Figure S6B).
3.4 S. aureus induces expression of keratinocyte-derived cytokines
To further understand S. aureus-mediated inhibition of ELOVLs, HEKs were differentiated and stimulated with S. epidermidis, MSSA, or MRSA for 8 and 20 h. S. epidermidis exposure did not affect HEK cytokine profile (Figure S7). MSSA significantly induced gene expressions of TNF-α and IL-1β after 8 h of stimulation (Figure 4A). At the same time, a significantly increased gene expression of TNF-α (p < .0001), IL-1β (p < .0001), IL-36γ (p < .001), IL-6 (p < .0001), IL-33 (p < .0001), IL-1α (p < .01), IL-8 (p < .001), and thymic stromal lymphopoietin (p < .001) were observed in HEKs stimulated with MRSA for 8 h (Figure 4B). Gene expression of TNF-α (p < .0001) and IL-1β (p < .0001) remained upregulated in HEKs stimulated with MSSA and MRSA for 20 h (Figure 4A,B). In addition, IL-36γ (p < .0001), IL-6 (p < .05), and IL-33 (p < .01) remained upregulated in HEKs stimulated with MRSA for 20 h (Figure 4B). Strain-dependent variabilities in TNF-α, IL-1β, and IL-33 mRNA induction were noted (Figure S8A–C).
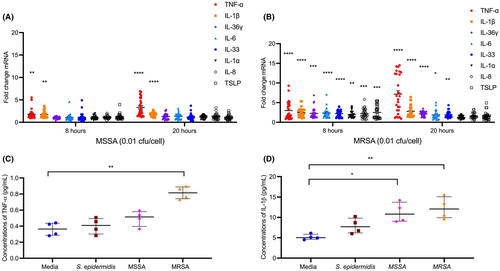
TNF-α protein levels were confirmed to be increased only in keratinocyte cultures stimulated with MRSA (Figure 4C) (p < .01, as compared to untreated cells). Protein levels of IL-1β were significantly increased in culture supernatants from MSSA and MRSA stimulated HEKs (p < .05 and p < .01 compared to untreated keratinocytes) (Figure 4D).
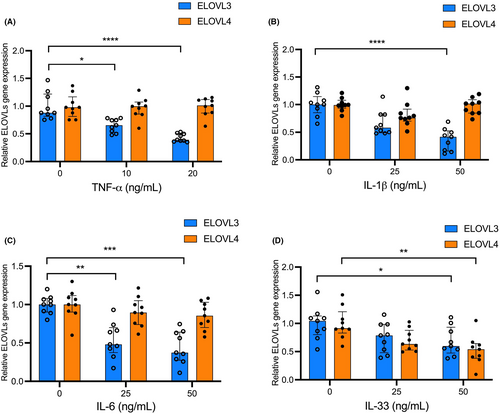
3.5 Keratinocyte-derived cytokines inhibit fatty acid elongase expression
To examine whether S. aureus-induced, keratinocyte-derived cytokines inhibit ELOVL3 and ELOVL4, HEKs were Ca2+ differentiated and stimulated with various concentrations of S. aureus-induced cytokines for 24 h. Gene expression of ELOVL3 was significantly inhibited in HEKs treated with TNF-α (Figure 5A), IL-1β (Figure 5B), IL-6 (Figure 5C), IL-33 (Figure 5D), and IL-36γ (Figure S9A) compared to cells treated with media alone. In contrast, ELOVL4 was only significantly inhibited in HEKs treated with IL-33 (Figure 5D). IL-1α (Figure S9B) and IL-8 (Figure S9C) did not inhibit ELOVL3 and ELOVL4.
3.6 Neutralization of keratinocyte-derived cytokines attenuates S. aureus-mediated inhibition of fatty acid elongase expression
We have investigated whether inhibition of MRSA-induced cytokines will interfere with ELOVL3 and ELOVL4 expression by keratinocytes. HEKs were differentiated and preincubated with isotype control antibodies or neutralizing antibodies against TNF-α (0.1 μg/ml), IL-1β (10 ng/ml), IL-36γ (1.0 μg/ml), IL-6 (0.1 μg/ml), and IL-33 (1.0 μg/ml) separately or in combination for 3 h, followed by stimulation with MRSA (0.01 CFU/cell) or MSSA (0.01 CFU/cell) for an additional 20 h. ELOVL3 gene expression was restored in HEKs stimulated with MRSA and individually added neutralizing antibodies against TNF-α (p < .01), IL-1β (p < .05), IL-6 (p < .05), or IL-33 (p < .05) compared to cells stimulated with a combination of MRSA and isotype antibodies (Figure 6A). Gene expression of ELOVL3 was fully restored (p < .05) in HEKs stimulated with MRSA combined with all neutralizing antibodies (against TNF-α, IL-1β, IL-6, and IL-33) (Figure 6B).
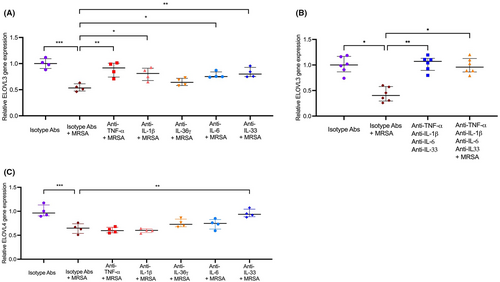
Gene expression of ELOVL4 was only restored in HEKs stimulated with the combination of MRSA and anti-IL-33 neutralizing antibody compared to cells stimulated with the combination of MRSA and an isotype antibody (Figure 6C). MRSA-mediated inhibition of ELOVL4 was not significantly altered by neutralizing antibodies, including anti-TNF-α, anti-IL-1β, anti-IL-36γ, or anti-IL-6 antibodies (Figure 6C). Additionally, MSSA-mediated inhibition of ELOVL3 expression was significantly attenuated by a combination of TNF-α and IL-1β neutralizing antibodies (Figure S10A,B). However, ELOVL4 gene expression was not inhibited by MSSA (Figure S10C).
4 DISCUSSION
CERs play fundamental roles in barrier function by taking a major part in the formation of multi-lamellar layers in the SC.18, 25 CERs are a complex family of sphingoid-based lipids which include a long-chain base and a FA in their structure.26, 27 FAs in mammalian cells are usually between 12 and 24 carbon chain length,28-31 and in some tissues can be as long as 34 carbon chain length.25, 26 It is known that the reduction of FA chain length correlates with a decreased skin barrier function, and very LCFAs (VLCFAs) with carbon chain length more than C24 are crucial for maintaining barrier function in the skin.26, 28, 32 The chain length of FAs in CERs, SMs, and LPCs is reduced in the skin of AD patients11, 30 and is associated with skin barrier dysfunction in these patients.5, 33 It has been recently shown that inhibition of ELOVL3 and ELOVL6 by IL-4/IL-13 in AD skin results in accumulation of palmitic and stearic acid in CERs, blocks FA elongation in VLCFA, and contributes to impaired skin barrier function.26, 27, 34 Our study is in agreement with previously reported decrease in VLCFA CERs in adult AD skin colonized by S. aureus16 and shows that these changes in FA chain length can also be observed in SMs and LPCs. However, the mechanisms of S. aureus contribution in the regulation of FA metabolism in the AD skin have not been established.
In our study, nearly 43% of S. aureus isolates from the lesional skin samples of AD pediatric patients were MRSA. This is consistent with previous studies that reported 10%–42% MRSA isolates among S. aureus-positive AD skin cultures.8, 35, 36 In AD skin cultures, MRSA strains are on the rise and are more virulent than MSSA.37-39 Consistent with these reports, in our study MRSA-colonized AD patients had higher AD severity and increased TEWL compared to MSSA-colonized AD patients. Importantly, we found that MRSA colonization is strongly associated with decreased VLCFAs (>C22) and increased C16-18 fatty acids in AD skin. However, MSSA colonization was weakly associated with abnormal lipid profiles. Although our organotypic skin model does not fully represent real human skin, which contains numerous microbes, we demonstrated that MRSA, not MSSA, caused aberrant lipid profiles in organotypic skin cultures, and MRSA exposure resulted in increased TEWL in these cultures.
We demonstrated that both MSSA and MRSA inhibited ELOVL3 expression, which is involved in elongation of C16-C22 fatty acids.25, 26 This was more pronounced in MRSA than MSSA-stimulated skin cultures. Furthermore, MRSA also inhibited ELOVL4, which is responsible for the synthesis of very VLCFAs (≥C26).25, 26 Importantly, our in vitro experiments were designed to mimic staphylococcal colonization, not infection, as the keratinocytes were exposed to the low dose of bacteria (0.01 CFU/cell), and low degree of cytotoxicity was observed after 24 h of bacterial exposure. These in vitro data support observations in the skin of the MRSA-colonized AD pediatric patients, as in these patients a significant increase in lipid species with palmitic and stearic acids and a significant decrease in lipid species with VLCFAs was observed.
Importantly, in the present study, we report that S. aureus, particularly MRSA, indirectly causes deficiency of ELOVLs and abnormal epidermal lipid profiles subsequently through S. aureus-induced cytokines. We found that MRSA induces IL-1β, TNF-α, IL-6, and IL-33 production by keratinocytes, while only increased IL-1β protein production was found in MSSA-stimulated keratinocytes. These findings are supported by the previous reports that S. aureus induces production of various cytokines,40-44 and these cytokines are highly expressed in AD skin.45-48 Intriguingly, we determined that TNF-α, IL-1β, IL-6, and IL-33 inhibit ELOVL3 expression in HEKs, while IL-33 inhibited both ELOVL3 and ELOVL4 expression. Strain-dependent variability in cytokine induction and ELOVL inhibition were noted. MSSA and MRSA strains that inhibited ELOVL3 production induced IL-1β, TNF-α, or both. MRSA strains that inhibited ELOVL4 production were the only strains that induced IL-33 production in keratinocyte cultures. These data suggest that the genetic variabilities and the differences in toxin production among MSSA and MRSA strains may influence their effects in keratinocytes.
We have established that S. aureus causes the deficiency of ELOVL3 and ELOVL4 by the action of S. aureus-induced cytokines. We showed that neutralizing antibodies against TNF-α, IL-1β, IL-6, and IL-33 can prevent S. aureus-mediated inhibition of ELOVL3 and ELOVL4. Particularly, IL-33 induction by MRSA is critical to cause abnormal lipid compositions because IL-33 downregulates both ELOVL3 and ELOVL4, which is responsible for the synthesis of VLCFAs (≥C26).25, 26 This may also explain why MRSA caused more detrimental effects on epidermal lipid profiles and skin barrier function than MSSA.
It has been reported that ELOVL3-ablated mice exhibit a defect in water repulsions and increased TEWL.49 Moreover, ELOVL4-deficient mice showed depletion of CERs with VLCFAs and died within a few hours after birth due to severe skin barrier defects.50, 51 Therefore, S. aureus-mediated inhibition of ELOVL3 may result in skin barrier dysfunction, and MRSA-mediated downregulation of ELOVL4 causes more critical skin barrier abnormalities. These findings imply that the controlling S. aureus colonization, particularly MRSA, is crucial for clinical management of AD patients.
Previously, we have demonstrated that IL-4 and IL-13, which are expressed in AD skin, can be also induced by S. aureus in vivo33, 52, 53 and inhibit ELOVL3 and ELOVL6.11 To eliminate the effects of IL-4 and IL-13 on ELOVLs in our experiments, we stimulated organotypic skin cultures and HEKs with S. aureus and confirmed that HEK-derived cytokines, which were induced by S. aureus (including TNF-α, IL-1β, IL-6, and IL-33), can also inhibit ELOVL3 and ELOVL4. The specific mechanisms as to how HEK-derived cytokines inhibit ELOVL3 and ELOVL4 have not been elucidated. The involvement of nuclear factor-kappa B54 mitogen-activated protein kinase pathway55 and signal transducer and activator of transcription-3 can be suggested as these pathways are activated by TNF-α, IL-1β, IL-6, and IL-33.56-58
S. aureus produces many virulence factors such as cytolysins, enterotoxins, PVL, and PSMs.59-61 It has been reported that various strains of MRSA are a more potent producer of PVL and α-toxin than MSSA.62-64 Moreover, it has been reported that PVL, PSMs, α-toxin, and δ-toxin carrying MRSA (USA300) induce production of TNF-α, IL-1β, and IL-6 in keratinocytes.65-67 We found that UV light-killed S. aureus and PVL do not inhibit ELOVL3 and ELOVL4, but PSMα3 induces TNF-α and inhibits ELOVL3 only. The contribution of these virulence factors in the regulation of the cytokine-mediated ELOVL inhibition remains to be determined. In this study, we propose that virulence factors from S. aureus do not inhibit ELOVLs directly but inhibit ELOVLs indirectly through S. aureus-induced cytokines because cytokine neutralizing antibodies abolished MRSA-induced inhibition of ELOVL3 and ELOVL4 in HEKs.
In summary, we report significant changes to epidermal lipids FA chain length in AD skin samples of pediatric patients colonized by MRSA. We confirmed that S. aureus-induced cytokine production by keratinocytes caused alterations in epidermal lipid composition and subsequent skin barrier dysfunction by inhibiting ELOVL3 and ELOVL4 in vitro. These changes were more pronounced after exposure to MRSA than MSSA. These findings suggest the clinical importance of S. aureus evaluation for the methicillin sensitivity in AD skin and emphasize that the control of S. aureus, especially MRSA, colonization is critical in managing patients with AD.
AUTHOR CONTRIBUTIONS
JK and BEK generated and interpreted data and wrote the first draft of the manuscript. EB, IB, and ASB analyzed lipid profiles. CFH, JHB LB, and JL performed cell cultures, ELISA, and RT-PCR. JB, SJK, YC, DH, and HYL analyzed and interpreted bacterial data. HYK, UL, MSK, HK, and JL recruited patients and obtained samples from patients. EG, KA, and DYML analyzed data, edited the manuscript, and obtained funding.
ACKNOWLEDGMENTS
This study was supported by NIH/NIAMS 5 R01 AR41256-28 grant, Alvogen, and funding (2021-ER120400-01, 2021-ER120400-02) from the Research of Korea Disease Control and Prevention Agency, Republic of Korea. The authors thank Pressure Biosciences, Inc., for a generous loan of Barocycler 2320EXT instrument to EB. The SMC-180307 and SMC-180145 were obtained from Asian Bacterial Bank (ABB) of the Asia Pacific Foundation for Infectious Diseases (APFID). The authors acknowledge Nicole Meiklejohn for her assistance in preparing this manuscript.
CONFLICT OF INTEREST
DYML has consulted for Regeneron, Sanofi, Genentech, Incyte, and Leo Pharma. EB had research contracts from LEO Pharma and SANOFI. The remaining authors do not have any conflicts to report.