The ingenious mast cell: Contemporary insights into mast cell behavior and function
Abstract
Mast cells are (in)famous for their role in allergic diseases, but the physiological and pathophysiological roles of this ingenious cell are still not fully understood. Mast cells are important for homeostasis and surveillance of the human system, recognizing both endogenous and exogenous agents, which induce release of a variety of mediators acting on both immune and non-immune cells, including nerve cells, fibroblasts, endothelial cells, smooth muscle cells, and epithelial cells. During recent years, clinical and experimental studies on human mast cells, as well as experiments using animal models, have resulted in many discoveries that help decipher the function of mast cells in health and disease. In this review, we focus particularly on new insights into mast cell biology, with a focus on mast cell development, recruitment, heterogeneity, and reactivity. We also highlight the development in our understanding of mast cell-driven diseases and discuss the development of novel strategies to treat such conditions.
Abbreviations
-
- BTK
-
- Bruton's tyrosine kinase
-
- CTMC
-
- Connective tissue mast cells
-
- CU
-
- Chronic urticaria
-
- CSU
-
- Chronic spontaneous urticaria
-
- HαT
-
- Hereditary alpha tryptasemia
-
- MC
-
- Mast cell
-
- MCp
-
- Mast cell progenitor
-
- MCAS
-
- Mast cell activation syndrome
-
- MMAS
-
- Monoclonal mast cell activation syndrome
-
- MMC
-
- Mucosal mast cells
-
- PMD
-
- Piecemeal degranulation
-
- SCF
-
- Stem cell factor
-
- SG
-
- Secretory granule
-
- SYK
-
- Spleen associated tyrosine kinase
1 INTRODUCTION
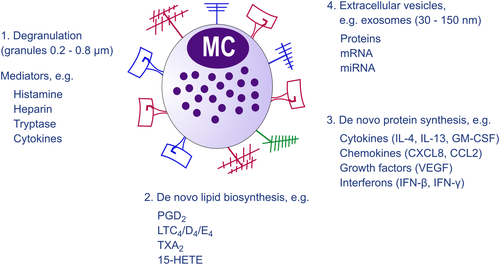
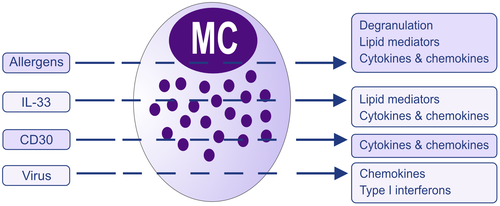
Although hematopoietic stem cells produce mast cell (MC) progenitors, mature MCs are normally absent in blood while found in almost all tissues as highly granulated cells. The stem cell factor (SCF) receptor, KIT, is one of the most critical receptors on mature MCs, as a reduction in KIT signaling leads to MC deficiency. Yet, signaling through the receptor is redundant for early development of MC progenitors in peripheral blood.1 MCs are equipped with a great number of receptors enabling them to sense and react to a diversity of stimuli.2 The most studied receptor that causes MC activation is the high-affinity IgE receptor, FcεRI. Another receptor that has received particular attention during recent years is the Mas-related G protein-coupled receptor X2 (MRGPRX2), a G protein-coupled receptor recognizing a variety of pharmacologic agents (tubocurarine, atracurium, icatibant, ciprofloxacin) causing pseudo allergic reactions.3, 4 MRGPRX2 also serves as a receptor for substance P, components of insect venom, antimicrobial peptides, secreted eosinophil products, and other cationic peptides.5, 6 MC expression of MRGPRX2 has been associated with neurogenic inflammation, pain, and itch 7-9 and also to contribute to the development of allergic inflammation.10 Other receptors that induce IgE-independent MC activation include the IL-33 receptor, which is important for MCs to recognize cell injury and trauma11 as well as regulating IgE-mediated responses,12-14 and pattern recognition receptors (PRRs) sensing “danger“ signals, including microbes.15 When MCs sense an endogenous or exogenous agent through binding to one of their many activating receptors, they react by releasing mediators through three major pathways (Figure 1). The most rapid response is exocytosis of secretory granules (SGs) and the release of preformed mediators such as histamine, proteases, and heparin.16 This is accompanied by the de novo biosynthesis of lipid mediators, predominantly eicosanoids such as prostaglandin D2 (PGD2), the cys-leukotrienes LTC4, LTD4, and LTE4, and also other eicosanoids like thromboxane A2 (TXA2) and 15-HETE,17 as well as other lipid mediators such as platelet-activating factor (PAF) and sphingosine-1-phosphate (S1P).18 In addition, MCs also have the capacity to synthesize a number of cytokines, chemokines, growth factors, and interferons.19 Notably, release of de novo-synthesized mediators can take place without preceding degranulation (Figure 2).20 Examples of such are the effect of IL-33 that induces secretion of leukotrienes and cytokines, for example,21, 22 CD30 inducing release of only cytokines and chemokines,23 and certain viruses, for example, respiratory syncytial virus, inducing secretion of type I interferons and chemokines 15, 24 (Figure 2). Thus, MCs can be activated and produce lipid mediators and/or cytokines in the absence of detectable degranulation (by histology or measurement of granule mediators).25 Finally, MCs also secrete extracellular vesicles including exosomes. MC exosomes can transfer proteins, enzymes, RNA, and miRNA that can be taken up by other cells, either proximal to the secreting MC or located at distant sites26-29 (www.exocarta.org) (Figure 1). Notably, patients with systemic mastocytosis have increased levels of exosomes with an MC signature including constitutively activated KIT, enabling transfer of mutant proteins to other cells.30
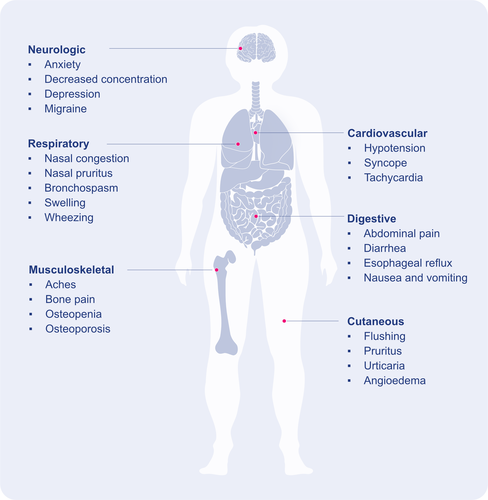
Given the broad distribution of MCs and their multifunctional role, the variety of mediators released, the symptoms associated with MC mediators (Figure 3), they have been implicated in many diseases beyond allergy.31-33 For example, recent reviews highlight the role of MCs in cardiovascular diseases,34 cancer,35 airway diseases,36 as well as in viral, bacterial, and fungal infections.37-39 Even if MCs commonly are discussed in the context of disease, it is important to remember that they also have a role in homeostasis, the initiation of acute inflammation,40 and in the protection against danger, whether it comes from the outside (venoms, pathogens, etc.) or from within the body (cell injury, etc.).41
In this review, we highlight some of the most recent findings regarding MC origin and development, recruitment, heterogeneity and reactivity, MC disease, new therapeutic possibilities, and animal models to study MC biology.
1.1 The origin of mast cells
Hematopoietic cells arise in several temporally distinct waves during prenatal development. The first MCs originate from primitive erythromyeloid progenitors in the extraembryonic yolk sac.42-45 A second wave of MCs appears together with the first definitive hematopoietic progenitors. The first two waves mainly contribute to connective tissue MCs (CTMC) and serosal-type MCs.43 The third hematopoietic wave originates from the aorta-gonado-mesonephros region. Cells formed during this wave produce hematopoietic stem cells that exhibit MC-forming potential.43 It was for a long time assumed that MCs develop from hematopoietic stem cells in the bone marrow.46 However, it is now clear that bone marrow hematopoietic stem cells produce a fraction, but not all, MCs. Experiments also show that bone marrow-derived MCs mainly replenish the mucosal MC population postnatally.43 However, there is a redundancy in the potential of the waves as bone marrow-derived MCs can reconstitute depleted skin-resident (connective tissue-type) MCs and populate the skin following inflammation.47
1.1.1 From hematopoietic trees to differentiation landscapes
The classic tree-like model that describes hematopoiesis assumes a stepwise loss of lineage potential with differentiation from hematopoietic stem cells to lineage-committed progenitors (Figure 4A). However, assignment of the MC differentiation trajectory to either the granulocyte-monocyte or the megakaryocyte-erythrocyte branch is a controversial topic. Studies supporting the idea that MCs belong to either branch have been presented.48-53 On a different angle, other investigations propose that MCs branch off early—close to hematopoietic stem cells—and differentiate along a unique trajectory.54, 55
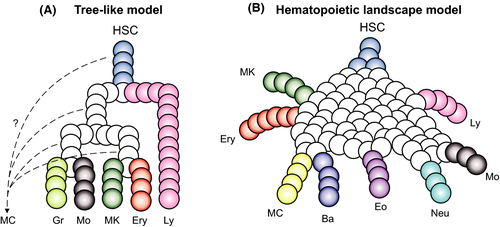
Advances in high-throughput single-cell RNA sequencing and computational biology are now delineating the MC differentiation trajectory by suggesting a revision of the hematopoietic model.56 The idea to reconstruct hematopoietic differentiation with single-cell transcriptomics is based on capturing a snapshot of single differentiating progenitors.57 Such analysis reveals that differentiating cells traverse a continuous landscape of states, from multi- and bipotent progenitors to lineage-committed cells (Figure 4B). A single-cell transcriptional landscape of bone marrow hematopoiesis in mouse shows that the MC developmental trajectory is positioned adjacent to the erythrocyte and basophil trajectories.51, 58 The MC and basophil trajectories are particularly close to each other,58, 59 which indicates the presence of a bipotent basophil-MC progenitor population, a finding that is recapitulated in single-cell fate assay experiments.49, 58-60 Cell fate assays of mouse bone marrow progenitors also verified that basophil/MC differentiation is closely coupled with erythropoiesis.51 The combination of single-cell transcriptomics and lineage tracing provides even deeper insights into hematopoiesis with focus on MC differentiation. Tracing individually barcoded mouse hematopoietic progenitor cells during in vitro culture confirms a coupling between MC and basophil fates as well as between MC and erythrocyte fates.61
In human, single-cell transcriptomics analysis of bone marrow cells indicates that basophils and MCs arise from a common bipotent progenitor.59 However, cell fate assays are yet to verify the existence of such progenitor. A link between basophil/MC progenitors and erythrocyte progenitors has also been proposed in human hematopoiesis.62-65 In analogy to lineage tracing experiment in mouse, tracking a somatic mutation in human hematopoiesis could potentially decipher the relationship between basophil and MC differentiation. The cellular distribution of KIT D816V mutation, a mutation present in systemic mastocytosis patients causing constitutive KIT signaling, has been studied for this purpose. No evidence supporting a close developmental relationship between MCs and basophils has been observed using this approach.66 In fact, the results based on charting the cellular distribution of the KIT D816V mutation seem to suggest that basophils and MCs are distantly related. However, such analysis rests on a disputable assumption; presence or absence of the somatic KIT D816V mutation in a given progenitor does not influence the cell's fate potential. Specifically, it is possible that the acquired D816 V mutation primes a multi- or bipotent progenitor toward the MC fate, given that KIT signaling promotes MC differentiation.67 This latter idea supports a close association between basophil and MC differentiation in health and explains the seemingly contradictory results in disease.
In summary, single-cell RNA sequencing has revolutionized our understanding of MC differentiation. The data reveal an overall landscape of hematopoiesis and an association between the MC, erythrocyte, and basophil granulocyte differentiation trajectories. The landscape model of hematopoiesis unifies old and new theories of the MC differentiation field and constitutes a foundation for future research.
1.2 Mast cell heterogeneity—how different are they from each other?
MC heterogeneity was first described in the mid-60 s by differences in histochemical staining features, and the concept of connective tissue mast cells (CTMCs) and mucosal mast cells (MMCs) was born.68, 69 In humans, different MC subpopulations have been defined by their protease content; those that express tryptase (MCT), tryptase and chymase (MCTC), and chymase only (MCC).70, 71 The latter was, for a long time, controversial, and the presence of chymase-only positive MCs has remained ignored and virtually unstudied. The two distinct MC subpopulations, MCT, and MCTC, as well as the MMC and CTMC, differ in their localization, mediator content, and responsiveness to secretagogues.72
Besides dividing MCs into subpopulations based on their localization or protease content, there are also definitions of MCs being either constitutive or inducible, inflammatory (iMC), or pro- or anti-tumorigenic (MC1 or MC2).35, 73-75 While the division into constitutive and inducible subpopulations might be related to the prenatal origin of the MC (see above), the latter is probably dependent on changes in the microenvironment during an inflammation. It is clear that MCs in different organs differ in their receptor and mediator expression, but also within a single tissue there is a considerable heterogeneity, for example, among human lung MCT.76 Furthermore, the gene/protein expression within this MCT subpopulation changes during inflammatory responses.75, 77 A question to address is if these changes relate to different subtypes or a plasticity within the MC population.
The origin and development of different MC subpopulations have been enigmatic, but one clue was described recently as mentioned above.45 For hematopoiesis in the adult, the question has been if there are several different MC progenitor populations in circulation, or if there is one progenitor population that has the capacity to differentiate and mature into any of the MC subtypes. In other words, is the heterogeneity driven by locally produced factors or is it driven by the recruitment of different types of designated progenitors? In a study where this question was addressed, the results suggested the existence of a common MC progenitor that gives rise to all MC subpopulations.78 However, single-cell RNA sequencing and single-cell cultures will likely provide further proof and insights into this process.
The separation of MCs into different subpopulations based on their protease content or histochemical properties is rather simplistic. MCs show a great plasticity, and detailed analysis of protease expression in human lung MCs demonstrate a gradient in the expression of the different proteases in these cells. Transcriptome comparisons of MCs from different mouse tissues with other immune cells revealed that MCs form a distinct population well separated from all other immune cells.79 Within the MC population, there was a substantial heterogeneity across tissue. Similarly, proteome analysis of human skin and fat MCs confirmed the unique identity of MCs among immune lineages, including the granulocytes (neutrophils, eosinophils, and basophils).80 In this study, there were few differences in the proteome between skin and fat MCs, raising questions on the impact of the microenvironment for MC heterogeneity.80 Analysis of single-cell RNA sequencing data of MCs from human tissues will further decipher MC heterogeneity and plasticity and might provide a clearer view about differences among MCs in different tissues, and also within the same tissue.
1.3 Mechanisms behind directions that mast cells take during their life cycle
1.3.1 Homing and migration
MC progenitors circulating in the blood, in humans defined as CD34+, KIT+, FcεRI+ cells,81 enter a specific tissue where they complete their maturation under the influence of locally produced factors. Surprisingly, the homing mechanisms for MC progenitors are still mostly unknown, except that it is a tissue-specific process.82-84 In addition to their basal homing, MC numbers clearly increase at sites of inflammation, such as in allergic rhinitis and asthma. This increase in MC numbers is likely a consequence of both increased proliferation, migration, and survival, as well as maturation of their progenitors recruited in response to chemoattractants.85, 86 The fact that some chemokine receptors, such as CCR1, CXCR2, and CXCR4, are expressed on both progenitors and mature MCs suggests that MC accumulation at sites of inflammation may also involve relocalization of mature MCs within a specific tissue.84-89
1.3.2 To migrate or to degranulate, that is the question
To avoid loss of their munitions before reaching their final destination at inflamed sites, mature MCs need to be regulated to migrate, but not yet degranulate90 (Figure 5). This is a challenging requirement as some of the MC chemokines induce degranulation of basophils91 and synergize with secretagogues to potentiate MC degranulation.92, 93 Thus, it was proposed that chemokines may either elicit distinct signals in MCs, as opposed to basophils or that MC SGs are linked to the cell cytoskeleton differently from the basophil granules.90 Marked differences observed between the actin skeleton in migrating versus secreting MCs support the latter possibility.94 Analysis of actin rearrangements following chemokine stimulation of MCs revealed an accumulation of pericentral actin clusters that prevent cell flattening and converge the SGs in the cell center.94 By contrast, reduction in the actin mesh density characterizes the secretory actin phenotype. Thus, the migratory actin phenotype immobilizes the secretory granules by trapping them in the cell center, whereas the secretory actin phenotype supports mobility and exocytosis. Diaphanous-related formin, mDia1, appears to be key player in these actin rearrangements94 (Figure 5).
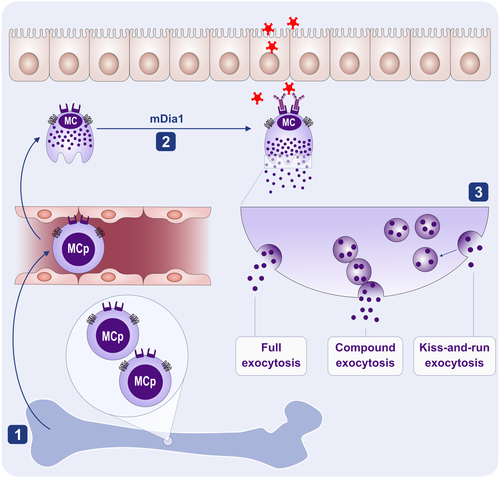
1.3.3 Kiss-and-run, full or partial exocytosis? The next question is how to degranulate
As MCs reach their destination and encounter a secretagogue, the secretory actin phenotype overrides the migratory actin phenotype, relieving the secretory constraints.94 MCs then enter a state in which they can undergo exocytosis (Figure 5). This can take place in three different ways: (i) individual SGs move to the plasma membrane where they dock and fuse with the membrane and release their full content; (ii) by compound exocytosis where SGs fuse with each other, forming a channel through which they release their content; or (iii) “kiss-and-run exocytosis” where SGs only transiently fuse with the membrane and release only part of their content.95 Choosing the mode of exocytosis bears physiological significance, as the distinct features and dynamics of the different exocytic modes are associated with unique physiological responses.96 This notion was nicely illustrated in a recent study that investigated the MCs’ response to innate stimulation (e.g., by the innate receptor MRGPRX2). Human MCs quickly mobilize discrete SGs toward the plasma membrane, resulting in very rapid secretion of individual SGs; by full or possibly also by kiss and run exocytosis.96 By contrast, activation of MCs by antibody-dependent adaptive stimuli (e.g., IgE + Ag), resulted in the much slower exteriorization of groups of granules formed by the prior intracellular fusion of individual SGs, resulting in compound exocytosis.96 Intriguingly, the distinct modes of exocytosis were linked with distinct physiological features. Thus, whereas the MRGPRX2-mediated trigger is brief and causes local inflammatory reactions, the IgE-stimulated reactions are more substantial and also lasted for a longer period of time.96
Ultrastructural studies of MCs have implicated an additional mode of secretion, which is independent of SG exocytosis.97 The latter, termed piecemeal degranulation (PMD), involves vesicle budding from the SGs and their transport and fusion with the plasma membrane. PMD has been associated mainly with MC activation under inflammatory conditions, including MC activation in pediatric cholelithiasis and biliary dyskinesia,98 functional dyspepsia,99and cancer.100 PMD could in principle lead to the selective release of some SG mediators, such as reported for secretion of serotonin without histamine from rat MCs.101, 102 However, the precise mechanism of such mediator segregation awaits further investigation. At the molecular level, two factors have been identified as critical for defining the degranulation pattern. The first is the enzyme IKKβ, whose activity is required for the phosphorylation of the SNARE protein SNAP23, which is essential for SG fusion during compound exocytosis.95, 96, 103 The second is the small GTPase Rab5, which plays a role in SG fusion during both their biogenesis104 and compound exocytosis.105 MCs may or may not release newly synthesized mediators, including lipid mediators, chemokines, and cytokines. Whether or not such release takes place is dependent on the stimulus type and may occur with or without prior release of preformed mediators.20 Thus, unlike the regulation of degranulation that takes place at the level of the exocytic process, regulation of cytokine and chemokine release occurs at their transcriptional level, whereas secretion utilizes the constitutive secretory and endocytic pathways.106 Mediators derived from arachidonic acid are immediately released, however, how precisely lipid mediators that are synthesized intracellularly are released is poorly understood.20 Finally, MCs release exosomes, whose content is dictated by the type of trigger.107
In conclusion, the underlying mechanisms that regulate MCs during their life cycle are only beginning to be clarified. They include mechanisms that ensure MC homing to destined tissues, recruitment to inflammatory areas, prevention of premature secretion, selection of the mode of exocytosis, and which lipid mediators, cytokines, or chemokines to synthesize de novo. Future studies will shed light on these mechanisms and the molecular entities involved.
1.4 Mast cells in diseases
MCs are involved in the initiation and perpetuation of a number of inflammatory conditions. These conditions range from those associated with an intrinsic or primary defect in MCs due to inherited or acquired polymorphisms and mutations within the MC compartment; to diseases where MCs are activated and recruited through an extrinsic mechanism such as formation of antigen-dependent and IgE-mediated MC activation; to clinical conditions where MCs are implicated through the release of MC mediators but where the mechanism of activation and recruitment is not well understood, thus “idiopathic” (Table 1).108
| 1. Primary (Intrinsic) |
|
a. Mastocytosis |
|
b. Monoclonal mast cell activation syndrome (MMAS) |
|
c. Autosomal dominant vibratory urticaria (VU) |
| 2. Secondary (Extrinsic) |
|
a. Allergic disorders (FcεRI mediated) |
|
b. Mast cell activation through the high-affinity IgG receptor (CD64) |
|
c. MRGPRX2-mediated reactions |
|
d. Diseases associated with complement activation (C3a, C5a) |
| 3. Idiopathicb |
|
a. Anaphylaxis |
|
b. Angioedema |
|
c. Urticaria |
|
d. Idiopathic mast cell activation syndrome (Idiopathic MCAS) |
There are several well-characterized molecular aberrancies affecting the MC compartment. Some are associated with recognized clinical diseases due to effects on MC proliferation and survival, MC reactivity, or MC mediator production. Mastocytosis is perhaps the most widely known disease associated with a primary defect in the MC compartment. It is a clonal disease involving expansion of tissue MCs. Mastocytosis is most commonly associated with an acquired gain-of-function KIT p.D816V missense variant, resulting in ligand-independent MC activation. This leads to both unrestrained growth of MCs and a lowered threshold for activation. Individuals with mastocytosis may present with flushing, pruritus, gastrointestinal complaints, or systemic anaphylaxis that may occur following exposure to Hymenoptera venom or for unidentified reasons. Mastocytosis may present as cutaneous disease only, or as a systemic disease with or without cutaneous manifestations.109
A group of patients with recurrent anaphylaxis has clonal MCs as demonstrated by evidence of one or two minor criteria for mastocytosis including aberrant MC morphology, CD25 expression, and/or presence of the KIT D816V point mutation. By consensus, such patients are currently said to have monoclonal MC activation syndrome (MMAS).110 If the marrow findings are observed in the absence of evidence of systemic MC activation, the term monoclonal MC disorder of uncertain significance has been suggested.
There are now a growing number of heritable genetic conditions that lead to increased MC reactivity. One such example is manifested as a physical urticaria. A missense substitution from cysteine to tyrosine (pC492Y) in the adhesion G protein-coupled receptor E2 (ADGRE2) is present in autosomal dominant vibratory urticaria (VU) characterized by localized hives and systemic manifestations in response to a local stimulus of frictional nature.111 ADGRE2 (CD312) belongs to a large family of adhesion GPCRs, generally with an extracellular domain facilitating interactions with proteins from the extracellular matrix. In this case, activation of MCs after a vibratory stimulus is evidenced by MC degranulation and an increase in histamine in the venous blood from the affected areas. Thus, ADGRE2 functions as a mechanoreceptor and induces cutaneous MC degranulation. Although the physiological relevance of the limited MC responses to friction in normal individuals is not completely understood, possibilities are that ADGRE2 may alert both resident and immune cells to combat potential injury and wound healing. It may also play a role in pain modulation and perhaps help sense a parasite migrating through dermal tissues.
Hereditary alpha tryptasemia (HαT) is a term used to describe a genetic trait caused by an increased TPSAB1 copy number encoding alpha-tryptase that leads to elevated basal serum tryptase levels in 4–6% of Western populations.112, 113 Individuals with multiple duplications of alpha-tryptase are reported to have a higher risk for severe anaphylaxis. Further, the prevalence of HαT in clonal MC disease is twice that of the general population and may be a biomarker for severe mediator-related symptoms in those with mastocytosis.114, 115 Recent mechanistic studies have demonstrated that unique enzymatic properties of alpha-tryptase containing heterotetrameric tryptases may contribute to this association.116 At present, HαT is thus best thought of as a disease-modifying genetic finding.
Extrinsic or secondary MC activation occurs primarily in allergic diseases, diseases associated with complement activation, and in association with activation of MCs through MRGPRX2. Symptoms may be infrequent to frequent and resultant disease sporadic or chronic depending on the activating mechanism. The immediate effects of MC degranulation, if localized to skin, include a wheal and flare reaction or, in airways, contraction of airway smooth muscle, mucus secretion, and an increase in vascular permeability. If systemic, the results may include severe hypotension and extensive vascular leakage. The early responses often transition into a late phase reaction hours later associated with an influx of circulating cell types which promote further inflammation.
The idiopathic MC category includes urticaria, angioedema, and anaphylaxis where there is no identifiable etiology, but where MC activation is documented through MC mediator release or evidence of MC degranulation in tissues involved (Table 1). The term idiopathic MC activation syndrome (Idiopathic MCAS) has been applied as a diagnosis for individuals who present with such episodic allergic-like signs and symptoms such as flushing, urticaria, diarrhea, and wheezing involving two or more organ systems, where the etiology is unknown.117 Diagnostic criteria include response to anti-mediator therapy and an elevation in a validated urinary or serum marker of MC activation, such as serum tryptase with an episode. Primary and secondary MC disorders must be eliminated as possible causes of the clinical findings. However, the search should continue for the etiology of these idiopathic disorders including the possibility that MC activation may relate to a yet-to-be-identified endogenous or environmental stimulus or an intrinsic MC defect resulting in a hyperactive MC phenotype.
1.5 The development of mast cell-targeted treatments
1.5.1 Mast cell mediators as targets of treatment
MC-targeted treatments are primarily developed for chronic urticaria (CU), mastocytosis, and allergies, because of the critical role that MCs play in their pathogenesis.118, 119 The evolution of MC-targeted treatments started, more than 70 years ago, with the development of drugs that inhibit the effects of individual MC mediators, first histamine, followed by prostaglandins and leukotrienes.120 Modern antihistamines that act on the histamine 1 receptor are superior to first-generation ones in their binding affinity, specificity, and risk/benefit profiles. Antihistamines that act on the histamine 4 receptor have shown promising results, and several such compounds are currently being developed for the treatment of MC-driven diseases.121 Recently a new approach to inhibit tryptase, the major MC protease, with an anti-tryptase antibody was tested in preclinical primate studies.122 The therapeutic area is initially severe asthma where MCs are implicated.123
However, to target one MC mediator or one of its receptors comes with an inherent limitation, that is, it prevents only the effects of one MC mediator or receptor. Activated MCs release many different mediators (acting on even more receptors) that are held to contribute to the development of signs and symptoms in patients with urticaria, mastocytosis, and other MC-mediated diseases.
1.5.2 Treatments that inhibit mast cell activation
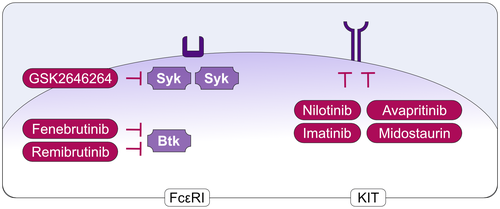
More recent attempts to target MCs therapeutically aim at the inhibition of activating signals and receptors (Figure 6). These include, for example, the high-affinity receptor for IgE, FcεRI, as well as the intracellular signals involved in translating receptor activation into degranulation and mediator release. Examples for the latter include Bruton's tyrosine kinase (BTK) and spleen-associated tyrosine kinase (SYK) (Figure 7). Inhibitors of BTK or SYK inhibit the degranulation of human MCs induced via FcεRI. Two BTK inhibitors, Fenebrutinib and Remibrutinib, as well as the SYK inhibitor GSK2646264, are currently under development for the treatment of patients with CU (Figure 7).
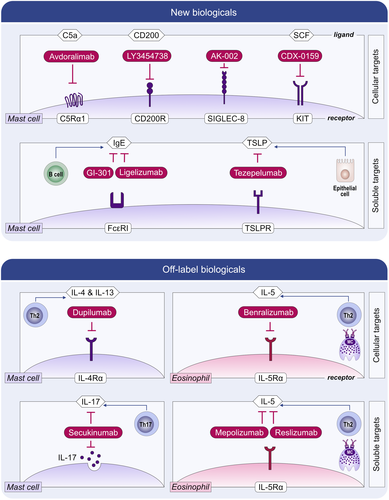
In chronic spontaneous urticaria (CSU), the activation of skin MCs via FcεRI, either by IgE to autoallergens or by autoantibodies to its alpha chain, is held to drive the development of signs and symptoms, itchy wheals and angioedema, in most patients.124-126 Treatment with omalizumab, an anti-IgE antibody, is effective in CSU127 and prevents MC activation by reducing the levels of free IgE and FcεRI expression. Newer anti-IgEs, including ligelizumab and GI-301, with higher affinity to IgE than omalizumab, are in clinical development.128
Several other activating signals and receptors are held to contribute to the activation and degranulation of MCs and, thereby, to the development of signs and symptoms of MC-driven diseases. These include the receptors for thymic stromal lymphopoietin (TSLP), IL-33, IL-4, IL-13, IL-5, and complement C5a as well as MRGPRX2. For all of these receptors, inhibitory compounds (or compounds that inhibit their ligands) are currently in clinical development for MC-driven diseases, primarily CU.129
The benefit of targeting MC-activating signals, receptors, and pathways is that this approach protects from the release and, therefore, the effects of all MC mediators rather than only one. The limitation is that only one of many pathways of MC activation and degranulation is shut down. Other pathways are untouched, remain viable, and can lead to MC activation, as they often do in most MC-driven diseases.
1.5.3 Treatments that silence mast cells
MCs express several inhibitory receptors.130 Their ligand engagement inhibits MC activation and degranulation, thereby silencing MCs. Inhibitory MC receptors, including Siglec-8 and CD200Ra, and therapeutic antibodies that engage them are currently under development for CU (Figure 6). The Siglec-8-targeted antibody AK002 inhibits MC activation (and depletes eosinophils) and showed promising results in the treatment of omalizumab-naïve and omalizumab-refractory CSU, symptomatic dermographism, cholinergic urticaria, and systemic mastocytosis. Several endogenous ligands for Siglec-8 have been identified. Siglec-8 preferentially binds the sialoside glycans 6′-sulfo-sialyl-Lewis X and 6′-sulfo-sialyl-N-acetyl-D-lactosamine. However, it is currently unknown how relevant they are for the inhibition of MCs in vivo.131, 132 The CD200Ra-targeted antibody LY3454738 is also under development for CU (Figure 6).
1.5.4 Treatments that reduce mast cell numbers
The KIT receptor is the key driver of MC differentiation, migration, proliferation, and survival. The inhibition of KIT or SCF leads to MC apoptosis and reduced MC numbers. Compounds that target KIT or SCF are currently explored for the clinical development in MC-driven diseases including CU and mastocytosis.133 For example, the antibody CDX-0159, which specifically binds the extracellular dimerization domain of KIT, was shown to induce profound and sustained suppression of plasma tryptase, indicative of systemic MC ablation (Figure 6). CDX-0159 is currently under development for CU. Examples for the development of oral KIT-targeted treatments include Imatinib, Nilotinib, Midostaurin, and Avapritinib (Figure 7), all of which show efficacy in mastocytosis linked to reductions in MCs and/or serum tryptase levels. To note, Imatinib and Nilotinib inhibit wild type KIT, but do not affect D816V-mutated KIT, and thus can only be used for D816V negative cases of mastocytosis. In addition, there are a number of potential targets, apart from KIT, that in experimental models have been shown to regulate MC numbers and activity.109, 133, 134
1.6 Animal models to study mast cell function
Numerous types of animal models, primarily in the mouse, have been utilized throughout the years to outline the contribution of MCs in diverse pathological settings. In the first generation of such models, MC deficiency in the mouse and rat was due to various mutations in Kit, that is, the receptor for SCF. Since SCF is an essential growth factor for MCs, defects in Kit result in an essentially complete absence of MCs. However, KIT is also expressed by a number of other cell types, and it has, therefore, been challenging to ascertain that consequences of Kit defects are indeed explained by an impact on the MC niche as opposed to off-target effects on other populations (reviewed in135, 136 ). To account for these issues, new mouse models of MC-deficiency, independent of Kit, have been developed (Table 2). These include mice where MC deficiency is driven by Cre recombinase expression under the control of MC-specific promoters. In one strategy, Cre recombinase was driven by the promoter for Mcpt5, a gene specifically expressed by CTMCs. These mice can then be crossed with R26DTA mice, leading to constitutive MC deficiency due to MC-specific expression of diphtheria toxin (DT).137 Alternatively, the mice can be crossed with the iDTR line, leading to MC-specific expression of the DT receptor; treatment of these mice with DT will thus lead to conditional depletion of MCs.137 In another approach, MC deficiency was accomplished by inserting Cre under the control of the promoter for Cpa3, a gene expressed predominantly by MCs.138 This leads to constitutive MC depletion, apparently due to Cre-mediated genotoxicity, but also to a substantial reduction of basophils, the latter in agreement with studies showing that basophils express low levels of Cpa3. The Cpa3 promoter was also exploited to generate a mouse strain in which the Cre-LoxP recombination system was used for deletion of the gene coding for the anti-apoptotic factor Mcl-1. This led to an essentially complete absence of MCs but also to a major reduction in basophils.139 MC depletion has also been accomplished in a model where the DT receptor gene was expressed under the control of an MC-specific IL-4 enhancer element.140 Another strategy was to insert the DT receptor and bright red td-Tomato fluorescent protein genes into the gene coding for the β chain of FcεRI, which is expressed by basophils and MCs. This approach can be used for the depletion of MCs and basophils and also as an elegant tool to visualize MCs/basophils in vivo.141 More recently, a mouse line with reduced numbers of MMCs (CTMCs were not affected) was generated by expressing Cre under the control of the baboon-α-chymase gene and crossing these to mice with a floxed allele of Mcl-1.142
| Model, designation | Principle | Type of MC affected | Basophils affected | Ref |
|---|---|---|---|---|
| Constitutive | ||||
| Mcpt5-Cre; R-DTA | Cre expression under the control of the Mcpt5 promoter; Cre-driven expression of DT | CTMCs | No | 137 |
| Cpa3Cre+ (“Cre-Master”) | Cre expression under the control of the Cpa3 promoter | CTMCs + MMCs | Yes (~60% reduction) | 138 |
| Cpa3-Cre; Mcl−1 fl/fl (“Hello Kitty”) | Mcl−1 deletion under the control of the Cpa3 promoter | CTMCs + MMCs | Yes (~60–80% reduction) | 139 |
| Chm-Cre; Mcl−1 fl/fl | Mcl−1 deletion under the control of the baboon-α-chymase gene | MMCs reduced; CTMCs normal | Not determined | 142 |
| Inducible | ||||
| Mas-TRECK | DT receptor expressed under the control of an MC-specific IL−4 enhancer element | CTMCs; MMCs not determined | Transient depletion; recovery of basophil populations 12 days after DT administration | 140 |
| Mcpt5-Cre; iDTR | DT receptor expressed under the control of the Mcpt5 promoter | CTMCs | No | 137 |
| Red mast cell and basophil (RMB) mouse | DT receptor and bright red td-Tomato fluorescent protein expressed under the control the β chain of FcεRI (Ms4a2) | CTMCs; MMCs not determined | Transient depletion; recovery of basophil populations 12 days after DT administration | 141 |
- Abbreviations: CTMC, Connective tissue-type MC; DT, Diphtheria toxin; MMC, Mucosal-type MC.
By using these Kit-independent models of MC deficiency, important insight into the biological function of MCs has been obtained. As expected, the use of Kit-independent mouse models for MC deficiency has firmly confirmed the essential role of MCs in allergic responses.138, 143 Moreover, recent studies have shown that MCs can have a role in melanoma dissemination,144 cutaneous lymphoma,145 collagen-induced arthritis,146 bone fracture-associated inflammation,147 and bone healing.148 It was also demonstrated that MCs aggravate osteoarthritis149 and can mediate the detrimental impact of smoke components on asthmatic features.150 Further, it has been demonstrated that MCs have a beneficial role in controlling bacterial clearance and promoting wound healing after Pseudomonas aeruginosa infection,151 whereas a detrimental impact of MCs was seen in skin infection by Sporothrix schenckii.152
However, it is important to note that the use of these novel mouse models for MC deficiency has challenged some previous findings where a contribution of MCs in various pathologies has been implied. For example, recent findings based on Kit-independent mouse models for MC deficiency have questioned the role of MCs in certain models for autoimmune diseases138 and obesity,153 as well as the proposed adjuvant activities of MCs.146 Hence, a more nuanced view of how MCs are involved in pathological settings is currently emerging.
In addition to the various mouse models described above, recent efforts have resulted in the generation of mice in which the MC niche is populated by human MCs. This was accomplished by transplanting human hematopoietic stem cells into NOD-scid IL2R-γ−/− mice, and then promoting MC growth by administrating plasmids expressing human SCF, GM-CSF, and IL-3. In these mice (denoted “humice” or NSG-SGM3), mature human MCs (co-expressing KIT and FcεRI) were detected in multiple tissues. These “humice” have so far mainly been used to study the role of MCs in anaphylaxis and cutaneous drug reactions,154-156 but also in the evaluation of new therapies, for example, using a BTK inhibitor.157 Moreover, functional human MCs could be developed from the bone marrow of these mice. Clearly, this humanized model has the potential to be used as a highly valuable tool to study human MC function. Another approach for studying the function of MCs is to evaluate mice deficient in various MC mediators, including the MC-restricted proteases, serglycin, and histamine (reviewed in 16, 123, 158).
2 CONCLUSION
MC biology and their function in health and disease have been a fascinating subject for many researchers over the years. Many seminal findings have been made since the discovery of MCs in the late 19th century (some of them given in Box 1), shedding light on the function of this ingenious cell. Nevertheless, there are many fundamental questions that still are awaiting an answer (some listed in Box 2). With the rapid developments in methodology, systems biology, etc., in combination with more relevant animal models, experimental human studies, and clincal investigations, we can foresee a rapid development in MC research in the coming years that will not only give insights about their biology but also a better understanding on their role in different diseases.
BOX 1. Major milestone discoveries in the field of mast cell biology
- 1863 Friedrich von Recklinghausen identify granulated cells in unstained connective tissues from different species.
- 1878 Identification and description of MCs by Paul Ehrlich159
- 1937 Heparin is present in MCs160, 161
- 1949 Description of systemic mastocytosis162
- 1953 MCs contain histamine163
- 1956 Demonstration of the involvement of MCs in anaphylaxis164
- 1966 Description of MC heterogeneity, that is, mucosal MCs and connective tissue MCs68, 69
- 1966 & 1967 Identification and characterization of IgE165-167
- 1970 & 1972 Slow reacting substance of anaphylaxis (SRS-A), later shown to be leukotrienes, was demonstrated to be released after IgE-receptor activation168, 169
- 1977 MCs develop from bone marrow cells46
- 1981 Identification of tryptase as the predominant MC protease170, 171
- 1982 Prostaglandin D2 shown to be released from MCs172
- 1987 MCs can express and secrete cytokines173, 174
- 1989 Characterization of the high-affinity IgE-receptor175, 176
- 1989 The central nervous system affects MC reactivity177
- 1990 Identification, cloning, and characterization of SCF178
- 1992 Demonstration that SCF is the main growth and maturation factor for human MCs67, 179-181
- 1995 Identification of the D816V KIT mutation in systemic mastocytosis182
- 2004 & 2006 MC proteases detoxify endogenous peptides and venoms183, 184
- 2015 Identification of MRGPRX2 as a receptor causing pseudoallergic reactions3
- 2018 Insight into the prenatal development of MCs43, 44
BOX 2. Future research perspectives
- • To determine if MCs from different hematopoietic waves exhibit distinct functions.
- • To determine the developmental relationship between the MC trajectory and other myeloerythroid lineages. Is it consistent in health and disease?
- • To identify mechanisms behind an increase in MCs at a site of inflammation: local proliferation, survival, and/or progenitor recruitment to the site of inflammation? What chemotactic factors drive MC progenitor recruitment in vivo?
- • To get a better understanding of MC heterogeneity in different tissues and to define the mechanisms that regulate MC plasticity.
- • To understand the mechanisms for differential release of MC mediators.
- • To decipher the contribution of MCs and their mediators in diseases beyond primary MC diseases and allergy.
- • To understand mast cell activation syndrome (MCAS). What are the mechanisms? How can it be diagnosed and treated?
- • Development of drugs that selectively target MCs.
- • Development of new KIT-independent MC deficient models (mouse, but also other species such as rat, ferret, and guinea pig that might be better for specific diseases), in which MC functions and contributions to health and disease can be specifically investigated.
ACKNOWLEDGMENTS
JSD, GP, and GN are supported by funding from the Swedish Research Council and the Swedish Cancer Society. DDM is supported by the Division of Intramural Research, NIAID, NIH. The authors apologize to the all colleagues who have contributed importantly to our understanding of mast cell biology and whose work has not been cited.
CONFLICT OF INTEREST
M. Maurer has received honoraria (advisory board, speaker) and/or institutional grant/research support from Allakos, Amgen, ArgenX, Astra-Zeneca, Bayer, Blueprint, Celldex, Celltrion, CSL Behring, Dr. Pfleger, FAES, Genentech, Gilead, GSK, Innate Pharma, Kyowa Kirin, Lilly, Menlo, MerckleRecordati, Moxie, Novartis, Regeneron, Roche, Sanofi, Third Harmonic Bio, MSD, UCB, and Uriach.




