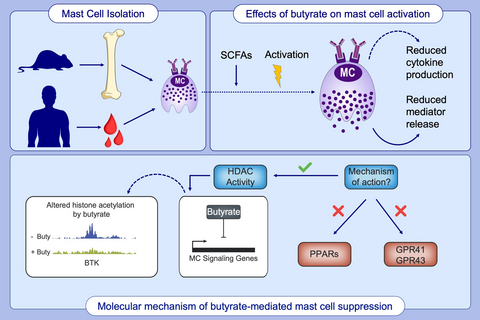
Butyrate inhibits human mast cell activation via epigenetic regulation of FcεRI-mediated signaling
Abstract
Background
Short-chain fatty acids (SCFAs) are fermented dietary components that regulate immune responses, promote colonic health, and suppress mast cell–mediated diseases. However, the effects of SCFAs on human mast cell function, including the underlying mechanisms, remain unclear. Here, we investigated the effects of the SCFAs (acetate, propionate, and butyrate) on mast cell–mediated pathology and human mast cell activation, including the molecular mechanisms involved.
Method
Precision-cut lung slices (PCLS) of allergen-exposed guinea pigs were used to assess the effects of butyrate on allergic airway contraction. Human and mouse mast cells were co-cultured with SCFAs and assessed for degranulation after IgE- or non–IgE-mediated stimulation. The underlying mechanisms involved were investigated using knockout mice, small molecule inhibitors/agonists, and genomics assays.
Results
Butyrate treatment inhibited allergen-induced histamine release and airway contraction in guinea pig PCLS. Propionate and butyrate, but not acetate, inhibited IgE- and non–IgE-mediated human or mouse mast cell degranulation in a concentration-dependent manner. Notably, these effects were independent of the stimulation of SCFA receptors GPR41, GPR43, or PPAR, but instead were associated with inhibition of histone deacetylases. Transcriptome analyses revealed butyrate-induced downregulation of the tyrosine kinases BTK, SYK, and LAT, critical transducers of FcεRI-mediated signals that are essential for mast cell activation. Epigenome analyses indicated that butyrate redistributed global histone acetylation in human mast cells, including significantly decreased acetylation at the BTK, SYK, and LAT promoter regions.
Conclusion
Known health benefits of SCFAs in allergic disease can, at least in part, be explained by epigenetic suppression of human mast cell activation.
Graphical Abstract
Short-chain fatty acids suppress primary human mast cell activation via HDAC inhibition and not via PPAR or GPR signaling. Butyrate induces transcriptional changes of genes important for signaling and activation of human mast cells. Butyrate triggers a redistribution of global H3K27 acetylation levels, leading to decreased acetylation at the transcription start site of genes encoding key FcεRI-mediated signaling molecules.
Abbreviations
-
- BMCMCs
-
- bone marrow–derived cultured mast cells
-
- GO
-
- gene ontology
-
- GPR
-
- G protein-coupled receptor
-
- HDAC
-
- histone deacetylase
-
- OVA
-
- ovalbumin
-
- PBCMC
-
- peripheral blood mononuclear cell-derived human mast cell
-
- PCLS
-
- precision-cut lung slices
-
- PPARs
-
- peroxisome proliferator-activated receptors
-
- SCFA
-
- short-chain fatty acids
-
- TSA
-
- trichostatin A
-
- TSS
-
- transcription start site
-
- WT
-
- wild-type
1 INTRODUCTION
Short-chain fatty acids (SCFAs) are positive regulators of immune responses and colonic health.1, 2 The three most prevalent SCFAs, acetate, propionate, and butyrate, produced by fermentation of nondigestible dietary fiber in the gut, are known to regulate gut integrity, colonic mobility, mucus production, and gastrointestinal pH.1, 2 SCFAs are important regulators of immune responses in human studies of IgE-mediated3 and non–IgE-mediated4 food allergy and mouse models of colitis, arthritis, and allergic airway disease.5 Although mainly produced in the gut, human and mouse studies indicate significant immunoregulatory effects of SCFAs in other tissues including the lungs,6-8 skin,9-11 and bones.12
Mast cells play a central role in initiating and maintaining inflammation, particularly in allergies and asthma, in which allergen re-exposure induces IgE-mediated FcεRI aggregation on the plasma membrane, rapidly triggering mast cell degranulation.15-17 Degranulation initiates the release of numerous inflammatory mediators13 and subsequent downstream signaling responses initiate the production of inflammatory cytokines, including tumor necrosis factor-alpha (TNFα)14, 15 and interleukin 6 (IL-6).16 FcɛRI aggregation induces phosphorylation of the Linker for Activation of T cells (LAT) adaptor molecule by the tyrosine kinases Lck/Yes-related Novel tyrosine kinase (LYN) and spleen tyrosine kinase (SYK).17 This signaling cascade subsequently triggers Bruton's tyrosine kinase (BTK) phosphorylation and activation of phospholipase (PLC)γ and protein kinase C, which increase the mobilization of calcium (Ca2+) to initiate mast cell degranulation.17
Mast cells are present in tissues where the body contacts the outside world,18 including the gastrointestinal tract, the upper and lower airways, and the skin. Due to their location in the gut and vascularized tissues, mast cells are exposed to high SCFA concentrations, reaching up to 140 mmol/L in the colon.23, 24 Notably, diffusion and active transport significantly reduce SCFA concentrations in human stool samples.3, 19 Importantly, SCFAs are also detectable in the blood20, 21 and previously implicated in protection against allergic airway inflammation, although the role of mast cells was not investigated.21
Previous studies have suggested possible links between fiber intake, SCFA concentrations, and mast cell–mediated pathology. Butyrate has been reported to decrease proliferation and increase histamine content in a mouse mast cell line.22 Butyrate also inhibits mouse mast cell degranulation and cytokine production23 as well as mast cell degranulation and inflammatory mediator content in the gut mucosa of piglets.24 However, the underlying mechanistic basis for these effects of butyrate remains unclear.25 Moreover, studies in primary human mast cells are lacking, which is critical since significant functional and phenotypical differences exist between rodent and human mast cells.26
Functional effects of SCFAs are often attributed to activation of membrane receptors GPR41 (or ‘FFAR3’) and GPR43 (or ‘FFAR2’),5, 27 the latter also expressed by mast cells.28 Nuclear peroxisome proliferator-activated receptors (PPARs) can also be stimulated by SCFAs, in particular butyrate.29 PPARs are expressed in mast cells30 and PPARγ stimulation attenuated atopic and contact dermatitis, possibly inhibiting mast cell maturation.31 Finally, butyrate is a known inhibitor of histone deacetylases (HDACs),32 a class of chromatin-modifying enzymes that play key roles in transcriptional regulation.33, 34 Butyrate inhibits class I and II HDACs, but not class III enzymes (including the Sirtuins).35 Dietary components, including SCFAs, were shown to promote gut homeostasis and immunity via control of histone acetylation and subsequent gene transcription.36, 37 Furthermore, the therapeutic use of butyrate to modulate gene expression has been employed for various diseases, including cancer38-41 and inflammatory disease.42-45 Notably, It was recently shown in mouse mast cells that butyrate suppressed FcεRI-dependent cytokine release, likely via inhibition of HDAC activity, without affecting β-Hexosaminidase.46
Here, we investigated the effects of SCFAs in a clinically relevant ex vivo model of mast cell–mediated pathology. Importantly, using primary mouse and human mast cells for functional assays as well as transcriptome and epigenome profiling, we have uncovered a critical mechanism of action by which butyrate suppresses mast cell activation.
2 METHODS
2.1 Animals
All protocols described in this study were approved by the University of Groningen Committee for Animal Experimentation, Groningen, the Netherlands. Guinea pigs were housed under a 12-hour light/dark cycle in a temperature- and humidity-controlled room with food and tap water ad libitum. Animals were actively IgE-sensitized to ovalbumin as described previously.72 The animals were used for experiments 4 weeks after sensitization.
2.2 Peripheral blood mononuclear cell-derived human mast cells
Human peripheral blood mononuclear cell-derived mast cells were generated as previously described by Gaudenzio et al.73 Briefly, peripheral blood mononuclear cells were obtained from buffy coats of healthy blood donors and CD34+ precursor cells were isolated using the EasySep Human CD34 Positive Selection Kit (STEMCELL Technologies). CD34+ cells were maintained for 4 weeks under serum-free conditions using StemSpan medium (STEMCELL Technologies) supplemented with recombinant human IL-6 (50 ng/mL; Peprotech), human IL-3 (10 ng/mL; Peprotech), and human Stem Cell Factor (100 ng/mL Peprotech). Thereafter, the cells were maintained in IMDM Glutamax I, sodium pyruvate, 2-mercaptoethanol, 0.5% BSA, insulin- 175 transferrin selenium (all from Invitrogen), ciprofloxacin (10 µg/mL; Sigma-Aldrich), IL-6 (50 ng/mL), and human Stem Cell Factor (100 ng/mL Peprotech). After 8-12 weeks, PBCMCs were tested for maturity by Giemsa or toluidine blue staining and beta-hexosaminidase release assays (see below).
2.3 Statistical analysis
Statistical tests were performed with Graphpad Prism 7 (GraphPad Software, Inc). Two-tailed Student's t tests (unpaired or paired) and one-way ANOVA tests were performed as described in the respective figure legends. A P < 0.05 was considered statistically significant.
A full description of all methods is available in the Data S1.
3 RESULTS
3.1 Butyrate reduces histamine release and inhibits OVA-induced airway narrowing
To mimic the mast cell–driven airway narrowing seen in asthma patients, we prepared precision-cut lung slices (PCLS) from the lower airways of guinea pigs sensitized to the model allergen ovalbumin (OVA). Subsequent OVA challenges induced airway narrowing in a concentration-dependent manner (Figure 1A). To assess the functional effects of butyrate on mast cell–mediated airway contraction, we treated PCLS with different concentrations of butyrate for 24 hours. Butyrate inhibited IgE- and allergen-induced airway contraction in a concentration-dependent manner (Figure 1A,B). Normal tissue viability and responsiveness were confirmed via a histamine challenge following the final OVA challenge (Appendix S1A).
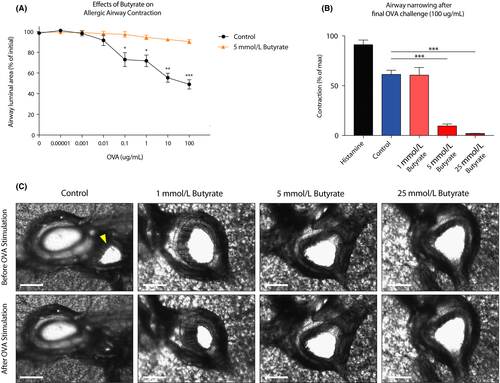
Histamine release by mast cells, and subsequent stimulation of the H1-receptor on airway smooth muscle cells, is a common inducer of airway contraction.47, 48 We measured histamine release in our ex vivo assay and found that OVA-challenged PCLS showed increased histamine release (Appendix S1B). Butyrate treatment of tissue slices abolished histamine release upon OVA stimulation (Appendix S1B). The concentration-dependent inhibition of airway narrowing by butyrate is visualized by representative photographs in Figure 1C and Videos S1-S4. Together, these data demonstrate that butyrate inhibits IgE-dependent mast cell activation in a clinically relevant model of allergen-induced airway narrowing.
3.2 Short-chain fatty acids propionate and butyrate inhibit primary mast cell activation
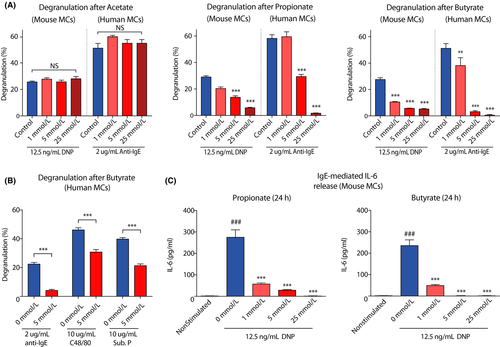
Given the continuous exposure of mast cell populations to high concentrations of acetate, propionate, and butyrate in vivo, we investigated the direct effects of these SCFA on the activation of cultured mast cells. Murine primary bone marrow-derived mast cells and human peripheral blood mononuclear cell-derived mast cells were incubated with increasing concentrations of the SCFAs acetate, propionate, and butyrate for 24 hours. Propionate and butyrate, but not acetate, inhibited IgE/antigen-induced mast cell degranulation in a concentration-dependent manner (Figure 2A). Propionate and butyrate—but not acetate—, at millimolar concentrations, induced up to 90% inhibition of both mouse and human mast cell degranulation after IgE/antigen-induced stimulation. Mast cell activation induced by ionomycin was also markedly inhibited by butyrate and, to a lesser extent, by propionate treatment (Appendix S2A), as was degranulation triggered by compound 48/80 or substance P (Figure 2B). In addition, mast cell secretion of IL-6, a typical inflammatory mediator produced by activated mast cells, was inhibited by both propionate and butyrate (Figure 2C). SCFAs did not affect total beta-hexosaminidase levels (data not shown), did not induce spontaneous mast cell degranulation (Appendix S2A) and did not induce cellular toxicity as measured by LDH leakage (Appendix S2B). Together, these data show that butyrate and propionate, but not acetate, potently inhibit both IgE- and non–IgE-mediated mast cell activation.
3.3 Inhibitory effects of SCFAs on mast cell activation are independent of GPR41, GPR43, and PPAR receptors
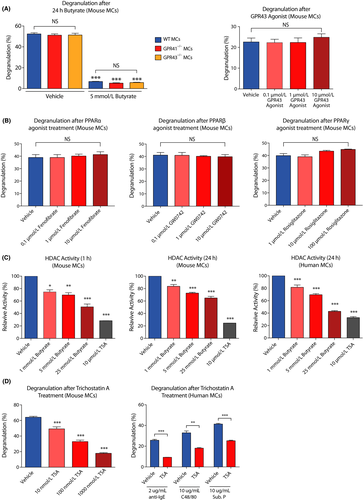
Next, we investigated whether the inhibitory effects of SCFAs depend on signaling through their known GPR41/43 and PPAR receptors. We found that FcεRI-mediated degranulation was highly similar in mast cells from GPR41−/− and GPR43−/− mice as compared to wild-type (WT) mast cells (Figure 3A). Importantly, GPR41 and GPR43 were not required for the inhibitory effects of butyrate (Figure 3A) or propionate (Appendix S3A,B) on mast cell degranulation. In line with these observations, direct agonist stimulation of the GPR43 receptor did not suppress mast cell degranulation (Figure 3A, right panel). Potential signal transduction of SCFAs via GPR109a was not further pursued due to very low expression detected in our gene expression analysis of primary human mast cells (see below; data not shown). Exposure of mast cells to PPARα, PPARβ, or PPARγ agonists also did not result in reduced mast cell degranulation (Figure 3B). Moreover, a specific PPARγ antagonist did not prevent the inhibition mast cell degranulation by butyrate (Appendix S3C). These results indicate that the effects of SCFAs on mast cell activity are not mediated via GPR41, GPR43, or PPAR receptor stimulation.
3.4 HDAC activity is regulated by propionate and butyrate and modulates mast cell degranulation
Short-chain fatty acids, and butyrate in particular, are known to act as inhibitors of HDAC activity. Indeed, administration of butyrate led to attenuated HDAC activity in both human and mouse mast cells (Figure 3C). Propionate also inhibited HDAC activity in mouse mast cells, albeit less potently than butyrate (Appendix S3D). In mouse mast cells, inhibition of HDAC activity was strongest after 1 hour of incubation (Figure 3C). Trichostatin A (or TSA, a potent pan-HDAC inhibitor) treatment also caused a significant decrease in human and mouse mast cell degranulation (Figure 3D), with a quantitatively similar impact as SCFAs (ie compared with Figure 2A). Therefore, SCFAs may regulate mast cell degranulation via modulation of HDAC activity and, as a consequence, gene expression.
3.5 Butyrate induces gene expression changes associated with cytokine signaling and activation of human mast cells
To assess whether butyrate treatment induced gene expression changes in primary human mast cells, we employed microarray gene expression profiling. The effect of butyrate on the human mast cell transcriptome was studied in nonstimulated mast cells and anti–IgE-stimulated mast cells. In nonstimulated mast cells, 1683 genes were differentially expressed (>twofold up- or downregulated) following butyrate treatment. Of these genes, 735 were upregulated (43.7%) and 948 downregulated (56.3%; Figure 4A).
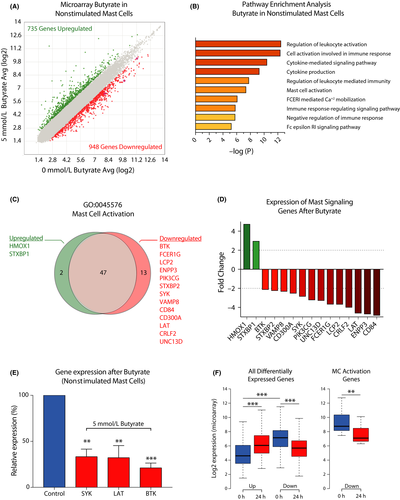
To investigate the cellular functions of butyrate-regulated genes and to link such functions to the regulatory effects of butyrate on mast cell activation, we performed a gene ontology (GO) pathway enrichment analysis. Human mast cells treated with butyrate for 24 hours predominantly showed expression changes in genes associated with the regulation of immune cell activation and (cytokine) signaling processes (Figure 4B). Butyrate treatment strongly affected transcription of genes associated with mast cell activation and FcεRI signaling. Of 62 genes, 15 linked to the mast cell activation pathway (GO:0045576) were found to be differently expressed, including reduced expression of the signaling genes BTK (-2.12-fold), SYK (-2.84-fold), and LAT (-4.6-fold), each of which is essential for full mast cell activation49-52 (Figure 4C,D). Downregulation of these essential genes following 24 hours of butyrate treatment was validated using qPCR in human mast cells from 3 additional donors (Figure 4E). Accordingly, 24 hours of butyrate exposure reduced BTK and SYK protein levels in mouse mast cells (Appendix S4A,B). The butyrate-induced transcriptional changes in FcεRI signaling pathway genes and how they explain the impaired mast cell response is further visualized in Appendix S4C. To further define the roles of butyrate-targeted genes in human mast cell activation, we examined the basal expression levels of these genes. Interestingly, genes upregulated in response to butyrate treatment showed overall low expression levels in the vehicle-treated mast cells, while downregulated genes showed significantly higher expression levels prior to treatment (Figure 4F). The 13 downregulated mast cell activation genes in particular were highly expressed before butyrate treatment (Figure 4F, right box plot).
In human mast cells stimulated via IgE-crosslinking, butyrate treatment also induced a robust transcriptional response, with 1767 differentially expressed genes following butyrate treatment (1092 upregulated and 675 downregulated, see Appendix S5A). Similar to nonstimulated mast cells, gene expression changes were associated with mast cell activation and cytokine production (Appendix S5B). Although butyrate primarily induced transcriptional upregulation in stimulated mast cells, genes in pathways associated with the activation of immune responses (eg mast cell activation, FcεRI-mediated signaling, and cytokine production) were strongly downregulated (Appendix S5C,D). Butyrate-induced downregulation of key mast cell activation genes BTK, SYK, and LAT also occurred in stimulated mast cells (Appendix S5E).
Finally, our microarray analysis showed that butyrate treatment regulated expression of genes associated with asthma and bronchoconstriction (ALOX5, LTC4S, and IFNGR2),53-57 JAK/STAT signal transduction (JAK3, STAT6), cytokine receptors associated with different immune responses (IL2RG, IL1RL1, IL18R1, IL18RAP, and CRLF2) and the negative regulator of NFkB signaling, TNFAIP3 (Data S2—MicroArray_Genelist). Furthermore, expression of TET2, a gene associated with epigenetic regulation of mast cell proliferation and activation, was downregulated.58-61 Together, these data indicate that butyrate regulates the expression of genes associated with mast cell activation, inflammatory responses, and cytokine signaling.
3.6 Butyrate triggers elevated global H3K27 acetylation but decreased acetylation at the transcription start site of human mast cell activation genes
Acetylated histones are associated with active gene transcription and represent a major substrate for HDACs. To assess whether butyrate-induced changes in HDAC activity result in an altered histone acetylation landscape in mast cells, we performed ChIP-Seq analysis of H3 lysine 27 acetylation (H3K27Ac)—a well-characterized epigenetic modification that recognizes active promoters and enhancers62—on two independent butyrate (or vehicle)-treated primary human mast cell cultures. To capture the immediate early effects of butyrate treatment on the mast cell chromatin landscape, we analyzed H3K27Ac levels after 3 hours of treatment. In line with its inhibitory effect on HDAC activity, butyrate treatment rapidly triggered increased global H3K27Ac levels, both in terms of genome-wide acetylation coverage and enrichment peaks detected (Figure 5A,B, shown for the KIT gene, as an example in Figure 5C). These observations were largely independent of analysis parameter settings (Appendix S6A,B). Notably, 3 hours of butyrate treatment predominantly triggered de novo acetylation events, with only a small minority (~1%) of regions that completely lost H3K27Ac (Figure 5A). De novo acquisition of H3K27Ac induced relatively modest acetylation mostly outside of or between regions that were highly acetylated in vehicle-treated mast cells (Figure 5C; Appendix S6C-E). Thus, butyrate exposure has a rapid and substantial impact on global mast cell histone acetylation.
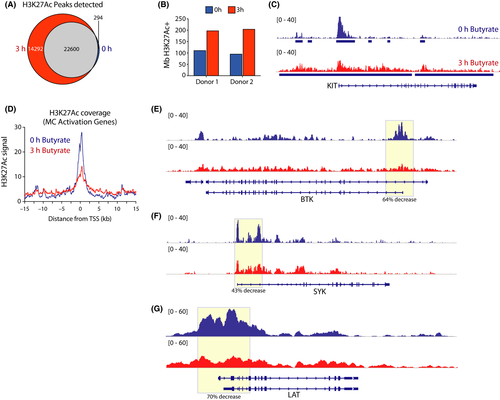
We next integrated H3K27Ac ChIP-Seq data with butyrate-induced transcriptional changes in nonstimulated human mast cells (see Figure 4). H3K27Ac levels around the transcription start site (TSS) of the 735 genes upregulated by butyrate treatment remained largely unchanged (Appendix S6D), although there was a minor reduction in peak height. In contrast, genes that were downregulated after 24 hours of butyrate exposure showed substantial deacetylation already at 3 hours of treatment (Appendix S6E). These included the set of 62 GO mast cell activation genes, which showed high H3K27Ac levels in the absence of butyrate, but were rapidly deacetylated around their TSS upon butyrate treatment (Figure 5D). Specifically, H3K27ac covering the TSS of BTK (64% decrease), SYK (43% decrease), and LAT (70% decrease) was substantially reduced (Figure 5E-G), correlating with their loss of expression upon butyrate treatment.
Together, these data show that exposure of mast cells to butyrate has a profound impact on their chromatin landscape. Butyrate evokes a low-level global histone hyper-acetylation, but also induces a specific loss of acetylated transcription-competent chromatin around highly expressed genes critical for FcεRI-mediated mast cell activation.
4 DISCUSSION
The immunomodulatory effects of SCFAs in mast cell-mediated disease, such as allergies and asthma, have been extensively studied. While SCFAs such as butyrate appear to suppress mast cell activity, the underlying mechanisms remain unclear and the direct effects of SCFAs on human mast cells have not yet been explored. Here, we validate that butyrate strongly reduces mast cell-driven airway narrowing in an ex vivo model of mast cell–mediated bronchoconstriction. These findings were further substantiated in a primary human mast cell culture system, as SCFAs propionate and especially butyrate inhibited both IgE- and non–IgE-mediated degranulation. Importantly, our studies in primary mast cells indicate that these effects are not dependent on the membrane receptors GRP41 and GPR43, nor on the nuclear PPAR receptors, which all have been implicated in mediating the biological effects of SCFAs. Instead, we show that HDAC activity in both mouse and human mast cells can be suppressed by propionate and butyrate, evoking a redistribution of global histone acetylation. Altered histone acetylation included a loss of acetylation and expression at genes encoding key signaling molecules mediating FcεRI-mediated degranulation, providing a plausible mechanism for how SCFAs can provide health benefits in the context of mast cell–mediated allergic disease.
The SCFA levels that we found to inhibit mast cell activation in vitro or ex vivo were nontoxic and comparable with physiological SCFA concentrations in the gut and blood of humans20, 63 or rodents.64 Although several mechanisms have been proposed to underlie the immunomodulatory effects of SCFAs,5, 27, 29, 30, 65 our data indicate that mast cell suppression by SCFAs is relayed via modulation of HDAC activity, rather than by the stimulation of membrane GPR41/43 or PPAR nuclear receptors. The noninstantaneous (>12 hours) effects of SCFAs on mast cell degranulation indicate that they are more likely to originate from alterations in gene expression, rather than by direct receptor stimulation. Indeed, we observed maximum HDAC inhibition directly after butyrate incubation (1 hour), followed by rapid changes in histone acetylation (3 hours) and a subsequent transcriptional silencing of FcεRI signaling genes for optimal suppression of mast cell degranulation (18-24 hours). Zhang et al recently reported that butyrate did not affect β-Hexosaminidase release in mouse mast cells. We believe that this discrepancy arises from the shorter exposure time (ie 12 hours) used in this study, which is likely not enough to allow for the abovementioned sequence of molecular events to sufficiently deplete the levels of FcεRI signaling components or other, possibly FcεRI-independent, mediators of degranulation.
Although butyrate-induced local histone deacetylation and subsequent transcriptional downregulation at highly expressed genes appears counterintuitive at first glance, earlier studies have also reported gene repression induced by HDAC inhibitors,66 particularly for highly expressed genes heavily occupied by HDACs.67-69 Of note, HDACs were reported to boost transcription by restricting histone acetylation specifically to the TSS.68 We postulate that HDAC inhibition by SCFAs triggers global ‘aspecific’ low level histone hyper-acetylation, especially near highly active gene loci that are preferentially targeted by HDACs. This redistribution lowers acetylation levels at transcription start sites, resulting in reduced mRNA expression, and histone acetylation at highly expressed genes (eg for BTK, Figure 5). Regulation of mast cell function by HDAC activity is further supported by a recent study demonstrating that TSA can diminish FcεRI-mediated cytokine production and degranulation in mouse mast cells.70
Our analyses suggest that a strong transcriptional silencing of critical molecules for IgE receptor-induced signal transduction, including BTK, SYK and LAT, represents the mechanism that underlies SCFA-mediated inhibition of human mast cell activation. Mast cells deficient in these signaling genes display reduced FcεRI-mediated degranulation.49-52 Importantly, BTK, SYK, and LAT are directly upstream of JNK and NFAT activation, potentially explaining the results of studies showing reduced JNK and NFAT phosphorylation or binding upon butyrate treatment.23, 24 Butyrate might additionally modulate AP-1 and NF-AT DNA binding through altered histone acetylation to suppress the late phase of mast cell activation. Whether butyrate exposure also modulates other (unknown) regulators of mast cell activity via histone acetylation levels is an important topic for future investigations.
The observation that butyrate prevented allergen-induced histamine release in PCLS of OVA-sensitized guinea pigs and markedly attenuated OVA-induced airway contraction indicates that SCFA-mediated HDAC inhibition of mast cell activation could potentially be of therapeutic interest in allergic diseases. Comparable effects were reported in a similar PCLS model using other HDAC inhibitors.71, 72 Notably, short-term butyrate treatment (2 hours) showed little effect on contraction of guinea pig PCLS,71 which parallels our findings that longer treatment with butyrate (24 hours) is needed for demonstration of inhibitory responses.
In summary, our findings indicate that SCFAs suppress human mast cell degranulation, cytokine production and allergen-induced airway contraction via HDAC inhibition and the subsequent transcriptional downregulation of critical mast cell signal transducers via an epigenetic mechanism. Hence, the acknowledged health benefits of SCFAs for allergic disease can be, in part, attributed to inhibition of mast cell activation via histone deacetylation. Additional insight into the inhibitory mechanisms of SCFAs may be of clinical importance and could reveal new approaches to inhibit pathogenic mast cell activity in allergic diseases.
ACKNOWLEDGMENTS
We thank Chen Liu for technical assistance, Reinoud Gosens, and colleagues (Department of Molecular Pharmacology, University of Groningen) for their advice and technical support in performing the PCLS measurements. JF is supported by a Fulbright Fellowship (financed by the Netherlands-America Foundation) and an EAACI Research Fellowship. RS is supported by an NWO Veni Fellowship (grant no. 91617114) and an Erasmus MC fellowship. This work was partly supported by the Lung Foundation Netherlands Grant 4.1.18.226 to RWH SJG, and SYT are supported by NIH/NIAID U19AI104209, NIH/NIAMS R01 AR067145, NIH/NIAID R01 AI132494, and United States-Israel Binational Science Foundation, Grant 2017182 (S. J. Galli).
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
J.F. performed the experiments with technical assistance of B.B., M.P.M.B., M.B., and W.F.J.I. and contributed to the data analysis. T.J. provided the bone marrow of GPR41 and GPR43 knockout mice. F.R., G.F., S.-Y.T., S.J.G., R.W.H., R.S., and M.M. supervised the projects and participated in experimental design and technical discussions. J.F. wrote the first draft of the manuscript. J.F., F.R., G.F., S.-Y.T., S.J.G., R.W.H., R.S., and M.M. drafted the manuscript.