Real-world evidence of subcutaneous allergoid immunotherapy in house dust mite-induced allergic rhinitis and asthma
Abstract
Background
The objective of this study was to analyze the effectiveness of allergen immunotherapy (AIT) with an allergoid in the treatment of house dust mites (HDM)-induced allergic rhinitis and/or asthma based on recent real-life data. The outcomes were measured using asthma incidence and consumption of corresponding medications as the indicator of persisting symptoms.
Methods
In this retrospective cohort analysis of a German longitudinal prescription database, patients who received at least two relevant mite AIT prescriptions in two different successive seasonal cycles were compared with non-AIT patients who received at least three symptomatic allergic rhinitis (AR) prescriptions in successive mite seasons. Study endpoints included AR progression, asthma progression, asthma occurrence, and therapy adherence. We used multivariate regression analyses to estimate the effects of AIT, adjusting for relevant variables.
Results
This study included 2350 patients receiving a mite allergoid and 64 740 control patients. After up to 6 years of follow-up, patients treated with mite allergoid required significantly fewer AR and asthma prescriptions (59.7% vs 10.8%) than the control group, and the probability of asthma development was significantly lower. The adherence of patients receiving allergoid was 63.8% at the end of the second year and 38.6% at the end of the third year.
Conclusions
This real-world evidence confirms the good efficacy of subcutaneous AIT with HDM mite allergoid in the treatment of allergic rhinitis and/or asthma. Up to 6 years of follow-up revealed significant effects in allergic rhinitis by measuring the number of AR medications and demonstrating significant reductions in asthma medications.
Graphical Abstract
In this retrospective, real-world study in Germany, effectiveness of (allergen immunotherapy) AIT with a subcutaneous (house dust mite) HDM allergoid was analyzed in comparison with a non-AIT control group. After up to 6 years of follow-up, the probability of asthma development in patients treated with HDM allergoid was significantly lower compared to the control group. Patients treated with HDM allergoid required significantly fewer medications for allergic rhinitis and asthma, thus indicating the preventive effect of HDM allergoid AIT on asthma occurrence and progression.
1 INTRODUCTION
Allergic rhinitis (AR) is a frequent chronic disease that seriously affects patients’ well-being and quality of life (QOL).1 European data found an average prevalence of 23% throughout Europe, ranging from 17% in Italy to 29% in Belgium, with an average of 21% in Germany.2
House dust mites sensitization is a high-risk factor in respiratory allergies, that is, allergic rhinitis and/or asthma.3 Allergic rhinitis is strongly associated with asthma.4, 5
In the European Community Respiratory Health Survey I, patients with allergic conditions had a mean prevalence of sensitization to HDM of 21.7%.6 Early sensitization to HDM in children <5 years of age is a significant risk factor for asthma later in childhood.7 HDM allergy is assumed to affect 1%-2% of the world's total population.8
The recommended methods for treating HDM allergy include avoidance of allergens, symptomatic medications, and AIT.5 Symptoms of AR can be reduced by symptomatic treatment using antihistamines, topical or inhalant corticosteroids (CS), and bronchodilators in asthma. AIT is effective as a long-term option and the only causative allergy treatment.9, 10
To evaluate the long-term effectiveness of mite AIT in a real-world evidence study setting, we chose an HDM allergoid as the most frequently prescribed SCIT product in Germany which has already been tested in several clinical studies.
A meta-analysis of three double-blind placebo-controlled clinical studies revealed that after 24 months of treatment, the HDM allergoid significantly improved the symptom medication score (Allergy-Control-SCORE™) in nonasthmatic mite allergic patients suffering from allergic rhinitis compared to placebo.11, 12 In another DBPC trial, the medication score was significantly reduced compared to placebo after treatment for 24 months.13 The preparation underlies the German Therapy Allergen Ordinance (TAO) process for gaining a marketing authorization.14 Within the TAO process, a dose range phase II trial with the Dermatophagoides pteronyssinus allergoid preparation in adults with well-controlled allergic bronchial asthma and allergic rhinitis (AR) or allergic rhinoconjunctivitis induced by HDM was conducted: after a 7-month course of SCIT, 23% of HDM allergoid treated patients reduced their ICS dose by two steps, the morning peak expiratory flow was improved by 41.99 L/min and the Asthma Control Test (ACT™) score by 5.45.15 In a randomized controlled trial in children suffering from asthma, a significant reduction of the minimum fluticasone propionate dose needed for asthma control was observed compared to a control group treated with standard asthma medication. After 2 years of treatment, morning peak expiratory flow increased significantly.16
Bronchial allergen provocation (BAP) is supposed to be an objective clinical tool to evaluate efficacy of AIT since it is not influenced by a placebo effect. The test is also suitable to discriminate between responders and nonresponders. In an open nonrandomized controlled trial in children and adolescents, the overall specific bronchial reactivity of the HDM allergoid treatment group improved significantly after 1 year of treatment whereas no change was detected in the control group. The responder rate was 60.7%.17 A recently published prospective study also using BAP for evaluation of efficacy of mite extracts in children and adolescents treated for 1 year with the HDM allergoid revealed a significant reduction in the maximum FEV1 fall between AIT pretreatment and the 1-year follow-up visit well as compared to an untreated control group. At the 1-year follow-up visit, 72.2% of patients significantly improved using the BAP test as evaluation parameter.18
Real-world evidence (RWE) data allow for estimates of effectiveness rather than efficacy in various typical practice settings, as well as examination of clinical outcomes in a diverse study population that represents patients observed in clinical practices.19 RWE is sparse with regard to the benefits of allergen immunotherapy. First data have been published covering AIT vs symptomatic medication use in patients with birch pollen-associated AR and/or asthma20 or grass pollen.21, 22
With regard to the impact of SCIT on patients suffering from HDM allergy, RWE data are not available. Therefore, our objective was to analyze the effectiveness of SCIT-based HDM allergoid on recent real-life data with regard to such outcomes as impact on AR medication, asthma incidence, and consumption of medications as an indicator of persistence in both adult and pediatric patients.
2 METHODS
2.1 Overall study design
The database used for the current analysis was IMS® LRx (IQVIA), which is based on about 60% of all German statutory healthcare prescriptions. The overall analysis period was January 2008 to February 2017.
The two strengths preparation Acaroid® contains house dust mite allergens extracted from purified mite bodies that are chemically modified and adsorbed onto aluminum hydroxide. It is standardized in therapeutic units (strength A: 1000 TU/mL; strength B: 10 000 TU/mL). The major allergen contents in the Dermatophagoides pteronyssinus preparation is 12 µgeq/mL Der p 1 and 10 µgeq/mL Der p 2; the major allergen content in the Dermatophagoides farinae preparation is 20 µgeq/mL Der f 1 and 15 µgeq/mL Der f 2.23 Recent data on high-resolution mass spectrometry (MS) proved that the preparation contains all 3 major and 4 intermediate mite allergens and 25 of 28 minor allergens.24
For AR, symptomatic medication consisting of nasal corticosteroids (NCS, ATC: R01A1) and antihistamines (ATC: R06A0) was chosen since these represent a fair share of prescription-only medications in Germany (Table S1). As of March 2017, a prescription was no longer required for NCS, so we only conducted the study up to February 2017.
The disease definition for AR was extended for the separate analyses of children alone since OTC medication is more likely reimbursed in such patients. Of the remaining ATC classes, nasal anti-allergic medications (ATC: R01A6) were also included in identifying AR. Furthermore, when analyzing the progression of this disease, ophthalmic products against conjunctivitis were added since rhinitis often occurs in association with conjunctivitis. The relevant classes included were ophthalmic corticosteroids (ATC: S01B0, S01C1) and, for the analyses of children, also anti-allergic ophthalmic preparations (ATC: S01G1-S01G3) (Table S2).
The identification of asthma was based on short-acting ß-agonists (SABA, ATC: R03A2, R03A4), inhaled corticosteroids (ICS) alone (ATC: R03D1) or combined with long-acting beta agonists (ATC: R03F1) and leukotriene receptor antagonists (ATC: R03J2) (Table S3). The asthma index definition was based on two relevant prescriptions since our intent in this study was to record the actual start of the disease. The asthma index was thus defined as the date of the first of two asthma prescriptions in the same or successive mite seasonal cycles.
2.2 Datasets and proxy clinical data
The longitudinal prescriptions database (LRx) collects pharmacy data from data centers where the prescriptions of all German patients with statutory health insurance are processed for reimbursement purposes. Data entries cover patient-specific data over time, including the patient's anonymized identification number, age, sex, insurance company, and region of living, as well as such prescription information as the prescriber's specialty, prescription date, and package information. The LRx database currently contains approximately 60% of all prescriptions reimbursed nationwide in Germany.25
German law allows the use of anonymous electronic medical records for research purposes under certain conditions. According to this legislation, it is not necessary to obtain informed consent from patients or approval from a medical ethics committee for this type of observational study that contains no directly identifiable data.
Because patients were only queried as aggregates and no protected health information was available for queries, no IRB approval was required for the use of this database or the completion of this study.
2.3 Indexing analytical time periods
Epidemiological data indicate that most patients with allergic rhinitis are polysensitized.26, 27 Since in this study HDM-allergic patients were defined based on symptomatic medication, it was important to restrict the identifying prescriptions to those in a period when medication would not be taken to relieve symptoms induced by other allergens. As the main pollen seasons cover February-April (early bloomers) and May-August (grasses), the indexing HDM exposition period was defined as September-January (next year). A HDM seasonal cycle thus covered the time from February to the following January, with the months from February to August constituting the preseasonal phase.
The intention was to evaluate the effect of AIT on AR and asthma medication consumption before vs at least 2 years after treatment. Therefore, patient history was divided into pretreatment, treatment, and follow-up periods. For the test groups, the treatment period ranged from the dates of the first mite AIT prescription (designated as the “index date” in the time between February 1, 2010, and January 31, 2014) to the expiry of the last such prescription. The 18 months before the index date represented the pretreatment period, and the entire interval after AIT, up to the release of NCS to the OTC market (28.2.2017), was designated as the follow-up period.
For the control group, the challenge was selecting a random segment of the patient's AR history while avoiding placing the index date at the beginning of this time period. The rationale for this was that AIT patients would not normally receive AIT if suffering from light AR and would have had a history of symptomatic AR treatment before the index date.
2.4 Patient inclusion/exclusion criteria
In order to make the two patient groups comparable, measures were taken to ensure a period of symptomatic treatment before the index date in both AIT and control patients. AIT patients (test groups) were selected based on at least two relevant mite AIT prescriptions in two different successive seasonal cycles. Patients in the control group were required to have had three symptomatic AR prescriptions in successive mite “seasons,” the second of these marking the index date. For both patient groups, a gap of at most one seasonal cycle between successive identifying prescriptions was permitted. The AIT patients were also required to have had a symptomatic prescription in the 18 months prior to the index date. All patients were selected on the basis of three prescriptions in 3-4 seasonal cycles. For the analyses of children only, the requirement for a symptomatic AR prescription before the index date was dropped.
Various exclusion criteria were applied to both patient groups. Only index dates that fell in the period 2/2010-1/2014 were accepted to ensure sufficient database observability before and after the treatment period. AIT patients switching between mite AIT products or receiving other forms of AIT (eg, against pollen) were excluded, and control patients were not permitted to have even a single AIT prescription of any kind in their history. Since the IMS® LRx database does not include ICD information, we included patients 5-50 years old on the index date to minimize the risk of including COPD patients. Patients with severe asthma (receiving anti-IgE or anti-IL5 biologicals) were excluded.
For the adherence analyses, patient selection was less stringent in order to also monitor patients with lower levels of adherence. Patients were included if they had a first prescription of relevant AIT medication in the index time window (2/2010-1/2014) with no such previous medication in the 730 days before the index date. Patients with a single focus prescription in the database were excluded.
2.5 Study endpoints
Study endpoints included the following: (a) AR progression based on a comparison of the annual number of symptomatic rhinitis or conjunctivitis prescriptions post-treatment vs preindex, (b) asthma progression based on the comparison of the annual number of asthma prescriptions postindex vs preindex in patients with asthma at baseline, (c) asthma occurrence, defined as the asthma occurrence after the AR index date in patients without asthma at baseline, and (d) adherence defined as the time from the first focus prescription to the expiry of the last focus prescription. Occurrence of a treatment gap between focus prescriptions (expiry of previous to dispensation of next) of more than 90 days or switch to another mite AIT product led to premature termination of the adherence period.
2.6 Statistical testing
The progression of AR and asthma were both tested using Poisson regression with a log link function and a dispersion factor, including confounding variables as covariates. The number of relevant prescriptions in the analysis phase was compared between the groups, while correcting for the patient-specific duration of this phase as one of the confounding variables.
The development of asthma among patients without the disease at baseline was assessed using logistic regression, adjusted for several covariates, including age, sex, prescriber specialty, region, duration of analytical period, and co-medication at baseline. We also corrected for the differential time after the index date between patients by including it as a covariate.
Adherence to therapy was tested between products and between different age groups within a product by using a log-rank test. This test shows any differences between at least two curves, so pairwise comparisons were conducted. The resulting P-values were corrected by Bonferroni adjustment to ensure that the overall type I/type II error remained at or below 5%.
The software used for statistical testing was SAS 9.4. All tests were considered significant at the 5% level (P < .05).
3 RESULTS
In total, 2 350 patients receiving HDM mite allergoid and 64 740 control patients (Figure S1) (analyses of children: 1 503 and 28 464, respectively (Figure S2)) were selected for the analyses. Their demographic and prescription-related characteristics at index are shown in Table 1. HDM allergoid patients were younger; female patients and patients treated by specialists were more often found in the HDM allergoid than in the control group. The follow-up period was slightly longer for the HDM allergoid than the control group (Table 1).
| Variables | All patients | Children | ||
|---|---|---|---|---|
| Allergoid | Control | Allergoid | Control | |
| Total patient count | 2 350 | 64 740 | 1 503 | 28 464 |
| Index age distribution (overall) | ||||
| 5-12 y | 715 (30.4) | 11 509 (17.8) | ||
| 13-17 y | 365 (15.5) | 3 725 (5.8) | ||
| 18-50 y | 1 270 (54.0) | 49 506 (76.5) | ||
| Index age distribution (children) | ||||
| 5 y | 57 (3.8) | 4 619 (16.2) | ||
| 6 y | 99 (6.6) | 3 963 (13.9) | ||
| 7 y | 122 (8.1) | 3 926 (13.8) | ||
| 8 y | 193 (12.8) | 3 843 (13.5) | ||
| 9 y | 233 (15.5) | 3 822 (13.4) | ||
| 10 y | 247 (16.4) | 3 314 (11.6) | ||
| 11 y | 257 (17.1) | 2 673 (9.4) | ||
| 12 y | 295 (19.6) | 2 304 (8.1) | ||
| Sex distribution* | ||||
| Male | 730 (49.7) | 18 481 (42.5) | 483 (56.8) | 9 667 (54.6) |
| Female | 740 (50.3) | 25 043 (57.5) | 367 (43.2) | 8 046 (45.4) |
| Unknown | 880 (37.4) | 21 216 (32.8) | 653 (43.4) | 10 751 (37.8) |
| Physician speciality distribution | ||||
| Generalist | 305 (13.0) | 38 284 (59.1) | 147 (9.8) | 8 202 (28.8) |
| Specialist | 2 045 (87.0) | 26 456 (40.9) | 1 356 (90.2) | 20 262 (71.2) |
| Number of years follow-up after AIT | ||||
| Mean (SD) | 3.43 (0.83) | 3.90 (1.21) | 3.40 (0.86) | 3.80 (1.21) |
| Asthma status at index date | ||||
| With asthma | 866 (36.9) | 27 007 (41.7) | 651 (43.3) | 12 236 (43.0) |
| Without asthma | 1 484 (63.1) | 37 733 (58.3) | 852 (56.7) | 16 228 (57.0) |
- * Sex distribution: male and female as % of patients with known sex, unknown as % of total.
3.1 Progression of allergic rhinitis
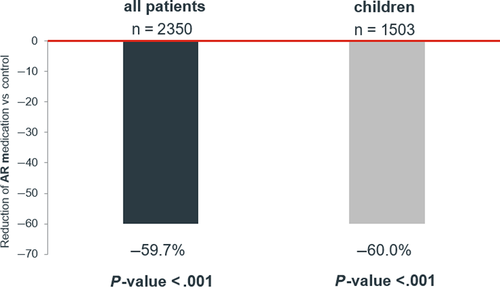
On average, patients who were treated with the HDM allergoid required 59.7% (95% CL: 55.3%-63.7%, P < .001) less number of prescriptions given to the control group after adjusting for all confounders. When only children were analyzed, this value was almost identical (60.0% (95% CI: 51.1-65.1) P < .001) (Figure 1).
3.2 Progression of asthma
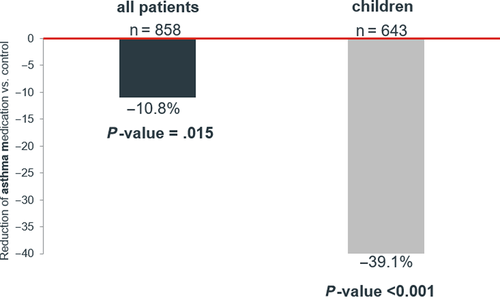
After up to 6 years of follow-up, HDM allergoid patients required 10.8% (95% CI: 2.2-18.6, P = .015) fewer asthma prescriptions than the control group. Among children, the reduction in medication prescriptions was 39.1% (95% CI: 32.1-45.4, P < .001) compared to the control group (Figure 2).
3.3 Asthma occurrence
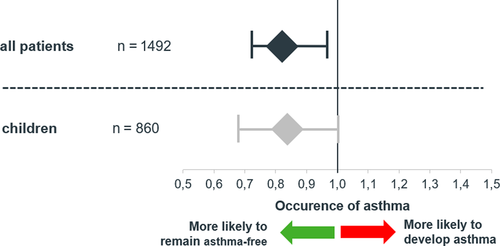
After covariate adjustment in the regression analysis, the probability of asthma development was significantly lower in the patients receiving HDM allergoid than in the control patients (odds ratio, OR = 0.81; 95% CI: 0.70-0.95) P = .008). Among children, the HDM allergoid patients also had a lower probability of developing asthma (OR = 0.83; 95% CI: 0.69-1.00, P = .052) (Figure 3).
3.4 Adherence
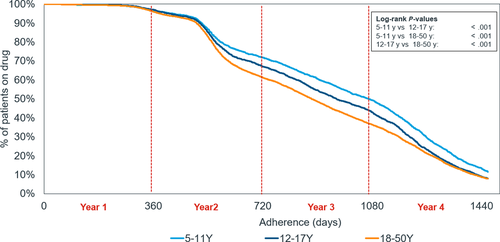
The adherence to HDM allergoid treatment was high throughout the first and into the second year (Figure 4). Adherence dropped more rapidly after around 500 days, reaching 63.8% and 38.6% at the end of the second and third years, respectively. When analyzed by age group, children had the highest adherence, followed by adolescents, and finally adults (47.9%, 41.3%, and 35.5%, respectively, after 3 years). The differences were statistically significant over the course of the analytical period (P < .001 for all paired comparisons).
4 DISCUSSION
Efficacy of AIT in HDM-induced AR and asthma has been demonstrated in randomized controlled clinical trials.16, 28, 29 However, the systematic evidence base is less clear when compared to AIT in pollen-triggered allergies.30 Furthermore, results need to be confirmed under long-term real-world conditions.31 Real-world evidence (RWE) data provide strong complementary information to RCT and support clinical treatment decisions by addressing real-world patient characteristics, treatment compliance, and long-term clinical outcomes (see Supplement Box 1).32, 33 To the best of our knowledge, our retrospective study is the first RWE study worldwide on the effectiveness of mite immunotherapy.
Real-world evidence studies comprise patient populations that are far more representative than those in RCTs and can include large sample sizes and provide information on treatment practices and outcomes in specific populations that are rarely included in DBPC trials, especially in groups of children.34 The IMS® LRx database as the underlying data source of this controlled retrospective cohort study comprises prescription data of 60% of all statutory prescriptions reimbursed nationwide in Germany. Though diagnoses (ICD information) are not available, disease states can be approximated based on disease-specific prescription data.22 Therefore, by adjusting for age, sex, physician specialty, number of seasons of AIT, AR treatments before AIT treatment, and region, we were able to report representative real-world outcomes of AIT with a high external validity.35
We included 2 350 mite allergy patients treated with the HDM allergoid and 64 740 control patients after applying all inclusion/exclusion criteria. 45% of all patients in the SCIT group were younger than 18, whereas the control group included 24% children and adolescents. An Italian real-world multi-center observational survey in 17 allergy clinics found a share of children of 25% in the AIT group. However, the authors stated a higher share in pediatric patients was to be expected, which is in line with our data.36
This study achieved a mean follow-up duration of 3.4 years for the HDM allergoid and 3.9 years for controls. By achieving a follow-up period of at least 3 years, a sufficient follow-up duration was reached to demonstrate the full potential of AIT effectiveness.37
House dust mites-induced AR is well known as a high-risk factor for asthma development.5 Our findings compare favorably with this observation, as we found a high share of asthma patients at the therapy index date (mite allergoid: 36.9%; control: 41.7%). These results are comparable to the Italian real-life data that showed that asthma was present in 41% of AIT-treated patients.36
This study demonstrates that AIT results in fewer symptomatic AR prescriptions than in controls. HDM patients treated with the HDM allergoid required only 40.3% (P < .001) of the number of prescriptions compared to the control patients. This finding is in line with results from a controlled 6-year trial of AIT in pollen allergy patients in Italy.38 In this study, the need for symptomatic drugs continuously decreased over time, whereas in AIT-untreated patients, the need for symptomatic medication remained unchanged over 6 years. A recent review on SCIT in HDM allergy patients concluded that SCIT was helpful in alleviating symptoms and reducing medication usage in HDM-sensitive asthma subjects.39
Several endpoints to prove efficacy in asthmatic patients have been tested in AIT trials: whereas Virchow et al focused on the effect on asthma exacerbations during ICS reduction Zielen et al demonstrated that children with house dust mite-induced allergic asthma could benefit from SCIT with the same mite allergoid used in this study by inducing a strong steroid-sparing effect while maintaining guideline-defined asthma control.16, 40 Our findings compare favorably with the published RCT data of the latter, as we were able to confirm that allergoid is significantly more effective at slowing asthma progression when compared to the control subjects. This effect is considerably more pronounced in children.
A long-term preventive effect of AIT in the development of asthma in children with AR up to 7 years after treatment completion was observed in a 10-year cohort study with SCIT.41 Again, our RWE outcomes support these findings, indicating that allergoid is significantly more effective in preventing asthma prescription intake compared to patients using non-AIT-based medications.
Adherence, which is characterized by wide variations in discontinuation rates, represents the most critical issue for the success of AIT as a treatment modality for allergic rhinitis.42-44 Satisfying compliance is particularly important to achieve both the immunological responses leading to clinical efficacy and the persistence of the clinical effects after completing AIT.37, 45 Furthermore, AIT should be administered for at least 3 years, which was confirmed in a recent analysis of health insurance data.46, 47 Our study obtained adherence data that differed for children, adolescents, and adults, indicating that children have the best adherence (50% after 3 years). Our RWE adherence results are, to some extent, better than average discontinuation rates based on European real-life data-based studies, including studies from Italy, Germany, Sweden, and the Netherlands.48 However, further improving adherence of AIT in HDM-allergic subjects remains a key factor in the real-world setting to achieve the maximum relief of patients’ disease burden. Patients’ involvement in the choice of AIT is an essential element in achieving this goal.37 Among patients previously receiving SCIT, 84% chose the same administration route, compared to 40% of patients with previous SLIT experience.3 Therefore, patient education, the provision of accurate information regarding adverse effects of AIT, and the use of regular (electronic) reminders are essential in real-world office settings.49
Overall, studies examining the pharmaco-economics of AIT have demonstrated cost savings.50 Furthermore, Kelly et al showed that corticosteroid-induced growth retardation in prepubertal children persisted even in adulthood, highlighting the need for reducing corticosteroid medication.51 Our results confirmed the potential of allergoid to reduce the need for asthma medication. A cost-effectiveness analysis by Reinhold et al quantified this effect, indicating that a mean increase of 35 L/min in morning peak flow (~10% of total peak flow) in SCIT-treated children can be achieved for an additional annual cost of about 385 euros with the HDM allergoid. The authors concluded that SCIT treatment in HDM was cost-effective.52
Our retrospective study based on nearly 70 000 patients represents the highest number of HDM allergy patients used for analyzing RWE effectiveness worldwide. Even if the data are derived from the real-world, therapy variables included in the analysis are unlikely to be biased. Furthermore, we applied a wide range of adjustment measures in order to reduce bias related to the uncontrolled nature of the data. Moreover, our analysis covers patients over several years, including pre- and post-treatment observation phases, with an average 1 148 days of treatment in children and 995 days in adults.
Since the present study used secondary data, some limitations should be mentioned. First, the database used does not include the diagnoses. Therefore, approximation of AR and AA, despite being meticulously specified with guideline-based treatment schemes, may not fully reflect the disease states being studied. Definition of AR patients by drug consumption may be difficult since, for example, ICS are also prescribed in patients suffering from chronic sinusitis or nasal polyps. This might also have led to a bias of more severe AR cases being included in the SCIT cohort, since allergic conditions will sometimes be treated on a trial-and-error basis in real-world office settings unless a convincing diagnosis exists.53 Furthermore, selection based on a period from September to the following January may have resulted in a slightly biased patient selection and not all patients with mite-induced allergic disease might have been detected. Though a peak in HDM allergy intensity is known to occur in fall and winter months, other relevant HDM allergy cases may not have been included. However, this selection time span prevented us from including non-HDM cases, such as pollen allergies. Nevertheless, our analysis may have also contained some patients in whom the prime allergic trigger was not HDM. Finally, due to the restriction that the database includes only reimbursed medications, OTC products are not covered in this analysis. Therefore, changes in patients’ overall drug consumption behavior could not be analyzed, assessment of AR progression may have been limited, and the treatment effect on reduction of AR medication may have been overestimated.
5 CONCLUSION
This study provides real-world evidence for support of data from clinical trials (RCT) demonstrating the significant efficacy of AIT in the treatment of HDM-induced AR and asthma. AIT is effective for up to 6 years in treating HDM AR measured based on relief medication consumption. Furthermore, our study found significant reduction in asthma medication use, indicating the preventive effect of the HDM allergoid on the occurrence and progression of asthma.
CONFLICT OF INTEREST
Dr Jutel reports personal fees from ALK-Abello, personal fees from Allergopharma, personal fees from Stallergenes, personal fees from Anergis, personal fees from Allergy Therapeutics, personal fees from Circassia, personal fees from Leti, personal fees from Biomay, personal fees from HAL, during the conduct of the study; personal fees from AstraZeneca, personal fees from GSK, personal fees from Novartis, personal fees from Teva, personal fees from Vectura, personal fees from UCB, personal fees from Takeda, personal fees from Roche, personal fees from Janssen, personal fees from Medimmune, personal fees from Chiesi, outside the submitted work. Dr Brüggenjürgen reports grants from Allergopharma, during the conduct of the study. Dr Vogelberg reports personal fees from IQVIA Commercial GmbH&Co. OHG, during the conduct of the study; grants and personal fees from ALK-Abello, grants and personal fees from Allergopharma, personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, personal fees from Bencard Allergy, personal fees from DBV Technologies, grants and personal fees from Novartis Pharma, personal fees from Sanofi Aventis, outside the submitted work. Mr Richter has nothing to disclose.





