Birch pollen allergy in Europe
Funding information:
Funding for this work was provided by ALK-Abelló, Hørsholm, Denmark.
Abstract
Birch and other related trees of the families Betulaceae and Fagaceae (alder, hazel, oak, hornbeam, chestnut, and beech) constitute the birch homologous group. This grouping is primarily based on the extensive IgE cross-reactivity of allergen homologs to the major birch allergen Bet v 1. Birch pollen is the most dominant tree pollen in Northern and Central Europe and is a major cause of allergic rhinitis and, possibly, asthma symptoms. Over the last few decades, levels of birch pollen have risen and the period of exposure has increased due to climate changes. Subsequently, the prevalence of birch pollen sensitization has also increased. The cross-reactivity and sequential pollen seasons within the birch homologous group create a prolonged symptomatic allergy period beyond birch pollen alone. Furthermore, many plant food allergens contain homologs to Bet v 1, meaning that the majority of patients with birch pollen allergy suffer from secondary pollen food syndrome (PFS). As a result, the negative impact on health-related quality of life (HRQoL) in patients allergic to birch pollen is significant. The purpose of this manuscript was to narratively review topics of interest such as taxonomy, cross-reactivity, prevalence, clinical relevance, PFS, and HRQoL with regard to birch pollen allergy from a European perspective.
1 INTRODUCTION
Pollen from birch and other related trees of the families Betulaceae and Fagaceae are the most dominant tree pollen types found in Northern and Central Europe and are a major cause of allergic rhinitis and, possibly, asthma symptoms.1-3 The broad cross-reactivity4, 5 and sequential pollen seasons of birch-related allergens6 prolong the period of allergic symptoms for many patients. In addition, cross-reactivity of birch pollen allergens extends to plant food allergens, resulting in the pollen food syndrome (PFS).7 Subsequently, the negative impact on health-related quality of life in patients allergic to birch pollen is substantial.8 The purpose of this manuscript was to narratively review topics of interest such as taxonomy, cross-reactivity, prevalence, PFS, and quality of life with regard to birch pollen allergy from a European perspective.
1.1 Birch taxonomy and homologous group
Birch trees belong to the order Fagales and family Betulaceae. In the 2016 taxonomic update from the Angiosperm Phylogeny Group, 6 other families are included in the order Fagales; the Integrated Taxonomic Information System also includes a 7th family (Table 1).9, 10 Of these families, trees within the families Betulaceae (birch, alder, hazelnut, and hornbeam) and Fagaceae (oak, chestnut, and beech) are commonly implicated in allergic rhinitis.11
| Families and genus (common name) related to birch by taxonomy9, 10 | Major pollen allergens | Species in the birch homologous group12, 15, 16 |
|---|---|---|
| Family Betulaceae | ||
| Alnus (alder) | Aln g 1 | Alnus glutinosa |
| Betula (birch) | Bet v 1 | Betula verrucosa |
| Carpinus (hornbeam) | Car b 1 | Carpinus betulus |
| Corylus (hazel) | Cor a 1 | Corylus avellana |
| Ostrya (hophornbeam) | ||
| Family Fagaceae | ||
| Castanea (chestnut) | Cas s 1 | Castanea sativa |
| Castanopsis (chinkapin) | ||
| Chrysolepis (chinquapin, chinkapin) | ||
| Fagus (beech) | Fag s 1 | Fagus sylvatica |
| Lithocarpus (tanoak) | ||
| Quercus (oak) | Que a 1 | Quercus alba |
| Family Myricaceae | ||
| Comptonia (sweet fern) | ||
| Morella (bayberry) | ||
| Myrica (sweetgale) | ||
| Family Juglandaceae | ||
| Carya (hickory, pecan) | ||
| Juglans (walnut) | ||
| Oreomunnea | ||
| Platycarya | ||
| Pterocarya | ||
| Family Casuarinaceae | ||
| Allocasuarina | ||
| Casuarina (she-oak) | ||
| Family Ticodendraceae | ||
| Ticodendron | ||
| Family Nothofagaceae | ||
| Nothofagus (southern beech) | ||
| Family Rhoipteleaceae | ||
| Rhoiptelea | ||
In 2009, Lorenz et al12 introduced the concept that allergen sources should be classified into antigenically related “homologous” groups based on comparable physicochemical and biological properties, as well as cross-reactivity toward the allergens of the different sources. The birch homologous group as suggested by Lorenz et al12 contains five tree species within the order Fagales (Table 1). These species are Betula verrucosa (European white birch), Alnus glutinosa (alder), Carpinus betulus (hornbeam), Corylus avellana (hazel), and Quercus alba (oak). Biochemical analysis has demonstrated that Bet v 1, along with the other major allergens in the suggested birch homologous group, are all 17 kD proteins of the pathogenesis-related protein class 10 (PR-10) family.13 There is a strong identity among the amino acid compositions of Bet v 1, Aln g 1, and Car b 1, and there is 79% to 83% amino acid sequence identity for Aln g 1, Cor a 1, and Car b 1 compared with Bet v 1.14, 15 Que a 1 demonstrates 58% amino acid sequence identity compared with Bet v 1.15 The European Medicine Agency has added Fagus sylvatica (beech) and Castanea sativa (chestnut) to the birch homologous group.16 The major allergens of beech (Fag s 1) and chestnut (Cas s 1) demonstrate a 69% and 75% (N-terminal) amino acid sequence homology with Bet v 1.15, 17 All of the major allergens of the birch homologous group also share structural homology and other biochemical characteristics with Bet v 1, although the molecular weight of Cas s 1 is 22 kD rather than 17 kD.15, 17, 18
The concept of “homologous” groups has been adopted by the European Medicine Agency in their Guideline on Allergen Products, which stipulates that to a limited extent, quality, efficacy, and safety data can be extrapolated from a representative allergen extract selected from one member of the homologous group to that of an extract within the same homologous group.16 Any extrapolation is also contingent on criteria of an identical formulation of the finished product and an identical production process of the allergen extract and of the finished product.16 Examples of the physicochemical and biological properties examined for similarity among the allergen extracts include protein, carbohydrate, lipid, and enzyme compositions, as well as water content.12
In the context of this manuscript, allergenic protein homologs are those relevant for patients suffering from allergic rhinitis to pollen from birch and other trees of the order Fagales.
1.2 Cross-reactivity of the birch homologous group
Cross-reactivity in immunologic terms refers to the ability of components of the immune system (ie, IgE antibodies and/or T cells) to recognize different antigens. Birch pollen allergens induce broad and complex patterns of IgE cross-reactivity, although cross-reactivity among allergens of birch pollen is predominantly observed in relation to the major allergen Bet v 1. Extracts of alder, hornbeam, hazel, oak, chestnut, and beech contain allergens that are homologs and cross-reactive in vitro to Bet v 1.4, 5, 14, 15, 19-21 Skin prick test (SPT) and radioallergosorbent test results support cross-reactivity within the birch homologous group.4, 5 The dominance of the birch pollen–derived allergens within the homologous group has been demonstrated in inhibition tests of tree-sensitive patients in Europe where a combination of recombinant Bet v 1 and Bet v 2 (a minor birch allergen) inhibited IgE binding to extracts from alder, hornbeam, hazel, and oak by an average of 72%-88%, indicating that together rBet v 1 and rBet v 2 contain 82% of the IgE epitopes present in Fagales pollens.5 Furthermore, the use of rBet v 1 in combination with natural birch extract has been demonstrated to identify patients with allergic rhinitis to homologous trees with a sensitivity of 99.2%.22
IgE cross-reactivity toward allergens within the birch homologous group needs to be considered when deciding on treatment. Based on the strong cross-reactivity of allergens within the order Fagales, allergen immunotherapy with extracts containing Bet v 1 theoretically could effectively cover sensitivities to all Fagales tree pollens.5, 23 In a clinical trial of a birch sublingual immunotherapy (SLIT) tablet, a significant improvement in the primary endpoint of a total combined symptom and medication score was observed versus placebo during both the birch pollen season and the total tree pollen season, which comprised hazel, alder, and birch.24 Clinical trials have demonstrated significant decreases in allergy symptoms versus placebo with immunotherapy products containing only recombinant or purified Bet v 1 in patients with birch allergy.25, 26
1.3 Prevalence of birch pollen allergy based on sensitization and symptoms
Birch pollen is a prominent elicitor of allergies in Europe. Of the birch pollen allergens, the prevalence of Bet v 1 sensitization is of the greatest clinical importance. In an Austrian study of 501 adolescents in the general population, 16.3% showed IgE reactivity to Bet v 1, and in a representative sample of 17 641 children and adolescents in the German general population, 14.1% were sensitized to Bet v 1.27, 28 In large cross-sectional studies of adults in the general population of Switzerland and Denmark, the prevalence of birch pollen sensitization was 7.9% and 13.7%, respectively.29, 30 A cross-sectional study of 2320 individuals in the Belgian general population reported a sensitization prevalence of 13.2% for tree pollen mix (birch, hazel, and alder).31 Thus, in general populations in Europe the prevalence of birch pollen sensitization ranges from approximately 8% to 16%. The prevalence of Bet v 1 sensitization is notably high among European patients with pollen allergies. In a study of 826 patients from the Czech Republic who were sensitized to at least one pollen allergen, molecular diagnostics using the chip technology (ImmunoCAP ISAC) indicated that 54.2% were sensitized to Bet v 1.32 In a study of 260 patients with tree pollen allergy in Germany, 239 (92%) were sensitized to Bet v 122 and in a retrospective study of 854 patients with birch pollen sensitization in Italy, sensitization to Bet v 1 ranged from 53% to 95%, depending on the region.33 These data indicate that birch pollen accounts for a large percentage of sensitizations among tree-allergic patients in the EU, with Bet v 1 being the major allergen.
Among tree pollen–allergic patients, sensitization to birch pollen without reactivity to Bet v 1 is uncommon and patients with IgE antibodies to birch pollen extract, but not to Bet v 1, will still present with reactivity to pollen extracts from hazel or alder.22, 34 In a study of 260 patients with tree pollen allergy in Germany, 239 (92%) were sensitized to Bet v 1, and of these 239 patients, all (100%) were co-sensitized to hazel and alder pollen extract.22 Only 21 (8%) of the tree pollen–allergic patients were not found to carry IgE to Bet v 1 using the ImmunoCAP assay as single test. However, of the 21 (8%) patients not sensitized to Bet v 1, 19 (90%) were reactive to birch pollen extract, all 21 (100%) were sensitized to alder pollen extract, and 10 (48%) were sensitized to hazel pollen extract.22 Rates of sensitization to minor birch pollen allergens are generally low, but can vary widely among different countries and regions (Table 2).22, 32, 33, 35 Sensitization to minor birch allergens may be a result of primary sensitizations to unrelated allergen sources such as grass pollen that contain protein homologs to Bet v 2 and Bet v 4 (ie, profilins and polcalcins).36 Bet v 2 and Bet v 4 sensitization appears to be more prevalent in southern regions.33, 35, 37
| Country | Birch-sensitive patients with specific IgE, % | ||
|---|---|---|---|
| rBet v 1 | rBet v 2 | rBet v 4 | |
| Finland | 100 | 2 | 5 |
| Sweden | 98 | 12 | 8 |
| Austria | 98 | 30 | 11 |
| France | 90 | 20 | 6 |
| Switzerland | 65 | 43 | 7 |
| Italy | 62 | 33 | 27 |
Over the last few decades, the level of birch pollen and pollen from the birch homologous group has increased in parts of Europe.38-40 This increase is due in part to the increased popularity of Betula as a decorative plant and also due in part to increases in overall temperatures.38-40 Subsequently, sensitization to Bet v 1 has increased in recent years. Results from 2 cross-sectional studies of the general population conducted in Northern Sweden found that sensitization to birch pollen increased from 13% in 1994 to 18% in 2009.41 Similarly, cross-sectional studies of the general population in Denmark reported an increase in birch pollen sensitization from 12.1% in 1990 to 13.7% in 1998.30 A small Finnish study evaluated IgE from the sera of birch pollen–sensitized patients and found that the prevalence of Bet v 1 sensitization increased from 29% in 1973 to 100% in 1994.42 Current studies now indicate that in some regions, greater than 90% of birch-sensitized patients have IgE against Bet v 1.22, 33, 35
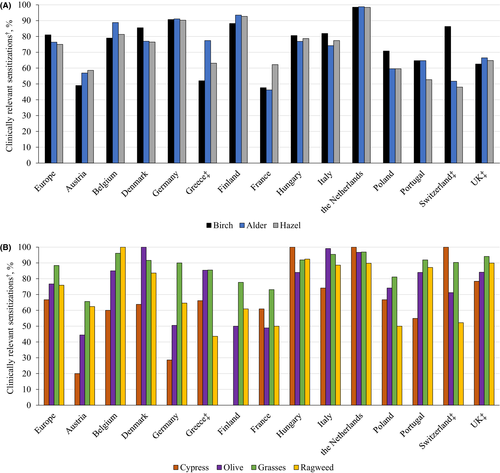
Although the homology of the major Fagales pollen allergens is very high and shows nearly 100% cross-reactivity in IgE binding,14 patients may react in varying degrees to birch and/or alder and/or hazel. In a European GA2LEN study, patients from 14 countries who were referred to allergy centers were tested for sensitization by skin prick test and clinical relevance was assessed by an allergist based on patients’ history of allergic rhinitis symptoms.43 Across European countries, the percentage of patients sensitized to birch, alder, and hazel ranged from 6.8%-57.4%, 3.1%-47.0%, and 7.4%-51.7%, respectively, and the percentage with clinically relevant symptoms ranged from 4.0%-49.1%, 2.3%-36.2%, and 3.9%-37.8%, respectively. The percentage who were both sensitized and who had clinically relevant symptoms ranged from 47.6%-98.5%, 46.2%-98.8%, and 48.0%-98.4%, respectively (Figure 1A).43 Other clinically relevant sensitizations to allergens such as cypress, olive, grasses, and ragweed are also found in varying degrees across Europe (Figure 1B).43
1.4 Pollen exposure for birch and homologous group trees
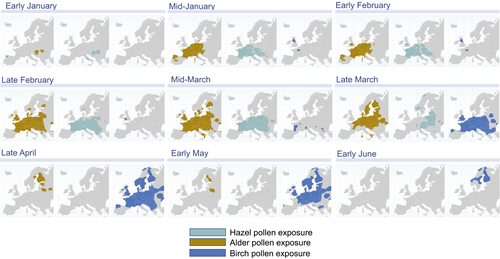
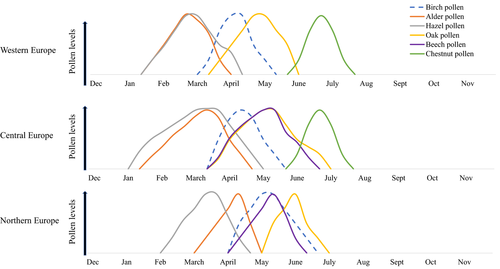
Trees in the order Fagales are found worldwide,11 although birch trees are most prevalent in Central and Northern Europe. Pollen seasons of the birch homologous group vary by species and latitude (Figures 2 and 3). In Western Europe, birch pollen season begins in early March and may extend through mid-May.44 Hazel and alder begin shedding pollen in January, followed by birch, oak, beech, and chestnut.6, 44-46 The timing of the pollen seasons is similar between Western and Central Europe, whereas the seasons are shifted approximately a few weeks later in Northern Europe (Figure 3).44-48 Because of the high cross-reactivity across the major allergens of the birch homologous group, and the broad time spectrum of the accompanying flowering seasons, the “birch” season extends well beyond the flowering of birch trees alone. It has been speculated that the early hazel and alder season may act to prime patients for birch pollen, followed by allergic responses to beech and chestnut pollen.6 Thus, birch pollen–sensitive patients may theoretically experience 2 to 3 months of pollen-related symptoms in individual regions, with the tree pollen season extending to up to 6 months when moving from one region to another throughout Central Europe and Scandinavia.
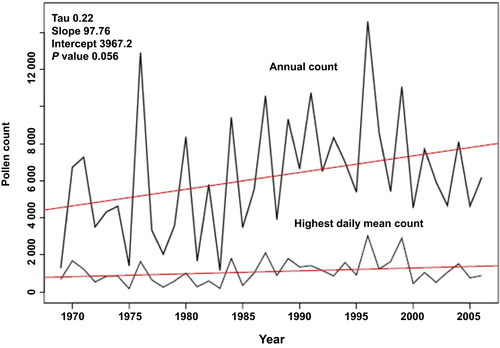
Climate change has had an impact on birch pollen over the last several decades. For example, warmer air temperatures can impact plant lifecycles by altering water and nutrient availability, soil type, and day length.49 A Swiss dataset spanning from 1969 to 2006 indicates that an overall temperature increase has led to an earlier onset of birch pollen season by about 15 days (Figure 4).39 Earlier start dates of the birch season have also been noted for London, Brussels, Stockholm, and Vienna.38, 48, 50 In addition, there has been a corresponding trend toward increased pollen concentrations associated with the overall warmer temperature.38, 39 Allergenicity may also be influenced by climatic conditions. In a study of birch pollen isolated from sources across Munich, Bet v 1 content was positively correlated with ozone levels and extracts prepared from high ozone sites induced significantly higher wheal and flare sizes in skin prick tests compared with extracts from low ozone sites.51
Substantial airborne birch allergens carried by small particles may be present even when birch pollen counts are low.52 Thus, outside of the active birch pollen (and those of homologous trees) season defined by pollen counts, birch pollen allergens are present in the air.52, 53 These atmospheric allergens may induce allergic rhinitis symptoms, but more research is needed to confirm this hypothesis. Furthermore, there has been documentation of ragweed pollen being transported by thunderstorms or wind currents hundreds or even thousands of kilometers into nonendemic areas, resulting in clinically relevant pollen levels.54-56 Modeling of birch pollen transport in Finland indicated the pollen sources included Baltic States, Russia, Germany, Poland, and Sweden.57 Therefore, birch pollen and/or birch allergens in the atmosphere may be transported to areas that are relatively birch-free (ie, Mediterranean regions) and account for the presence of birch pollen allergy in these areas. Further research into this hypothesis for birch pollen is needed.
1.5 Cross-reactivity between birch pollen and food items
Birch pollen–allergic patients frequently experience IgE-mediated allergic reactions upon contact with a large number of fruits, vegetables, roots, and nuts. These reactions are caused by IgE cross-reactivity with birch pollen allergens, namely Bet v 1 and its homologs, as demonstrated in IgE inhibition studies (Table 3).58-60 The degree of birch pollen–related food cross-reactivity is highly dependent on structural conformation and is determined by the epitope repertoire recognized by the specific IgE antibodies of individual patients.61 Some PR-10 food proteins like apple and hazelnut are more frequently recognized than others like soy, celery, and kiwi, indicating differences in the degree of homology.62
| Food | Bet v 1 Homolog |
|---|---|
| Almond | Pru du 1 |
| Apple | Mal d 1 |
| Apricot | Pru ar 1 |
| Carrot | Dau c 1 |
| Celery root/tuber | Api g 1 |
| Cherry | Pru av 1 |
| Hazelnut | Cor a 1 |
| Kiwi | Act d 8 |
| Peanut | Ara h 8 |
| Peach | Pru p 1 |
| Pear | Pyr c 1 |
| Plum | Pru d 1 |
| Soybean | Gly m 4 |
Approximately 70% of birch pollen–allergic patients experience these hypersensitivity reactions by IgE cross-reacting food sources.63, 64 For Bet v 1 sensitization, reactions are mainly in response to Rosaceae fruits (ie, apple), nuts (ie, hazelnut), and Apiaceae vegetables (ie, carrot).7 The prevalence of Rosaceae IgE sensitization in birch pollen–allergic patients is much higher than that of the general European population, in whom IgE sensitization to hazelnut and apple is 9.3% and 6.5%, respectively.65 Regional differences in the spectrum and relevance of food species are evident, perhaps due to variation in nutritional habits and/or selection bias.61 For example, studies of Austrian (N = 225) and Swedish (N = 380) patients with birch pollen allergy reported IgE-mediated reactions to apple in 80% and 47% of patients, respectively, 51% and 34% to peach, 41% and 26% to walnut, and 35% and 23% to carrot.63, 64
Symptoms of pollen-related food allergies typically comprise mild immediate local reactions such as itching, tingling, and angioedema of the lips, tongue, and throat. More severe reactions may include dysphagia or throat swelling, but patients may also experience systemic reactions such as urticaria, rhinitis, or anaphylaxis.58, 66 Thus, the initial general designation “oral allergy syndrome” for this phenomenon is somewhat misleading and has been renamed “the pollen food syndrome (PFS).” Although it may be commonly assumed that severe systemic reactions with pollen-related food allergy occur much less frequently than with non–pollen-related food allergy, severe reactions do occur.67
In Northern Europe, apple allergy is rarely found in the absence of birch pollen allergy because the major apple allergen Mal d 1 itself is inefficient or unable to induce IgE antibodies.68 All epitopes of the major apple allergen Mal d 1 are also present on Bet v 1.69 Together, these data support the hypothesis that Bet v 1 acts as the primary immunogen in most Northern European patients with PFS. In contrast, patients with apple allergy in Southern Europe tend to have cross-reactivity to other fruits, but without Betula pollen sensitization.70, 71 In such patients, the reactions to exposure tend to be systemic and more severe than PFS and involve allergens other than Bet v 1, most likely Mal d 3.70, 71
Food allergens that are homologs to Bet v 1 are generally considered the most frequent cause of clinically relevant pollen-related food allergies; in more than 90% of patients with birch pollen–associated food allergies, Bet v 1 is the primary associated birch allergen.7 However, in some regions a higher proportion of patients with PFS are sensitized to Bet v 2.33 The cross-reactivity toward Bet v 2 and food allergens is due to the presence of profilin proteins in food (Table 4). The probability of experiencing PFS is higher in polysensitized (ie, profilin-sensitive) than monosensitized birch pollen–allergic patients in these regions.72 Other predictors of PFS in patients with birch pollen allergy have also been identified. For example, birch pollen–allergic patients with PFS in general show higher birch pollen–specific and total IgE levels than patients without PFS.72-76 Furthermore, the presence of asthma and non–birch pollen respiratory allergies is more common in patients with PFS.72
| Food | Profilin Allergen |
|---|---|
| Almond | Pru du 4 |
| Apple | Mal d 4 |
| Celery root/tuber | Api g 4 |
| Cherry | Pru av 4 |
| Hazelnut | Cor a 2 |
| Kiwi | Act d 9 |
| Peanut | Ara h 5 |
| Peach | Pru p 4 |
| Pear | Pyr c 4 |
| Potato | Sola t 8 |
| Soybean | Gly m 3 |
| Walnut | Jug r 5 |
The presence of specific IgE to Bet v 1-homolog food proteins does not necessarily predict clinical symptoms after contact with the allergen source, which is a phenomenon often observed for allergens in general.77 Specific IgE against apple and/or hazelnut allergens in one study was detectable in sera from 47% of birch pollen–sensitive patients who did not have PFS.72 Similarly, 75% of birch pollen–allergic patients showed IgE-mediated cross-reactions to the Bet v 1-homolog soy protein Gly m 4 in vitro, but only 10% of the patients developed clinical symptoms after ingestion of soy products.78 However, the absence of IgE to Bet v 1-homolog food proteins is usually reliable.79 Uncertainty of the clinical relevance of SPT reactions to fresh food or food allergen extracts also exists, although birch pollen–allergic children with symptoms after eating fresh apple, carrot, or potato had significantly larger SPT reactions than children without a history of food allergy.80 The most reliable method for pollen-related food allergy diagnosis is blinded oral challenge.79
1.6 Clinical impact of allergy to birch pollen (and homologous groups)
In a birth cohort study of 764 Swedish children, the presence of IgE against Bet v 1 in early childhood (<4 years of age) was a predictor of allergic rhinitis by the age of 16 years.76 The overall odds ratio of having allergic rhinitis symptoms at age 8 or 16 years in children with Bet v 1 sensitization at age 4 was 7.1 (95% CI, 3.3-15.3).76 Symptoms associated with birch pollen allergy include nasal symptoms such as rhinorrhea, sneezing, and congestion, as well as eye symptoms such as watering and redness.81 In a cross-sectional study of the general population in Belgium, 81.1% of patients sensitized to a tree mix of birch, hazel, and alder experienced nasal symptoms.31 Based on diary data from voluntary patient input into a web-based app, the presence and severity of nasal symptoms and medication use in a region of Germany were found to correlate with birch pollen levels, particularly during peak season as defined by the European Academy of Allergy and Clinical Immunology (starts with 3 consecutive days of ≥100 pollen/m3).82 Thus, clinicians and patients may be able to anticipate the onset of peak birch-related allergy symptoms by monitoring pollen levels.
The role of birch pollen allergy in the development of asthma and asthma symptoms is still under debate. Whole birch pollen grains measure approximately 22 μm in diameter, which is too large to reach the lower airways.83 However, fine respirable particles of <7.2 μm in diameter that contain Bet v 1 are present in the atmosphere, particularly on days of light rain during the birch pollen season, and have the potential to trigger asthmatic responses in susceptible people.84 A significant correlation between respirable atmospheric allergen particles and asthma-related emergency department visits has been demonstrated for grass pollen,85 but the clinical implication of birch allergen–containing atmospheric particles on asthma has yet to be definitively demonstrated. In a population of pollen-allergic patients in Spain, a significantly higher proportion of patients sensitized to Betula pollen (42%) had asthma symptoms compared with non–Betula-sensitized patients (23%; P = 0.003).86 Furthermore, a study of 6 aeroallergen seasons (2008-2013) in Brussels found that an interquartile range increase in birch and hornbeam pollen resulted in a 3.2% (95% CI, 1.1, 5.3; P < 0.05) and 0.7% (95% CI, 0.2, 1.3; P < 0.05) increase in asthma-related hospitalizations.87 However, unlike grass pollen, exposure to birch pollen allergen did not have an impact on the lung function of children in a Swedish birth cohort.88
Patients with birch pollen allergy may also demonstrate worsening of atopic dermatitis (AD) symptoms approximately 6 to 48 hours after ingestion of cross-reactive food allergens.89-91 These late AD reactions in response to birch pollen–related food allergens appear to be mediated by T-cell cross-reactivity rather than IgE cross-reactivity.90, 91
Allergic rhinoconjunctivitis in general is well-known to have a negative impact on health-related quality of life (HRQoL). Surveys, questionnaires, and prospective studies reveal significant reductions in outdoor activity, sleep quality, emotional well-being, and work/school performance.92-94 Presumably, the same negative impact on HRQoL applies specifically to birch pollen allergy, although there is surprisingly little published literature to support this assertion. One published study that evaluated HRQoL in patients specifically allergic to birch pollen simply reported that there was no difference in HRQoL between monosensitized and polysensitized (defined as sensitization to at least 2 allergens among Bet v 1, Bet v 2, and Bet v 4) patients.95 Using the Rhinitis Control Assessment Test, another study found that 45.7% of patients with birch pollen allergy who were receiving treatment in accordance with Allergic Rhinitis and its Impact on Asthma recommendations had insufficiently controlled symptoms.8 More research is needed to determine the impact of birch allergy specifically on HRQoL, as well as the socioeconomic burden of disease.
There is a general perception that since the birch pollen season is short, birch allergy may be easily managed. However, as discussed above, the cross-reactivity within the birch homologous group, along with the varying pollen seasons, expands the potential for birch pollen–related allergy symptoms to be present for several months of the year. In addition, the birch pollen–related PFS exists beyond the tree pollen season63 and likely creates a burden on HRQoL as notable as the pollen allergy itself. Evaluation of food allergy–related HRQoL in German adults with birch pollen allergy and PFS revealed mild to moderate impairment, with the most severe impairment being in the Food Allergy-Related Health domain.96 Compared with men, women demonstrated a significantly greater negative impact in all domains measured, and older women (≥44 years of age) had a significantly greater impairment in the Risk of Accidental Exposure and Emotional Impact domains compared with younger women.96 The older women felt they had no control over their food and were a burden to their hosts, leading to increased apprehension about eating out. A study of children with PFS found that there was a moderate impact on all domains of the food allergy–related HRQoL questionnaire, with significantly more anxiety regarding time spent preparing food compared with patients without PFS.97
Numerous clinical trials have demonstrated the benefit of allergen immunotherapy via both subcutaneous and sublingual tablet routes of administration for birch-related allergic rhinoconjunctivitis.24-26, 98-102 Novel epicutaneous patch delivery systems for birch AIT are also under development.103 Considering the association between birch-related allergens and PFS, it was expected that AIT with birch extracts would be efficacious for PFS. To date, however, the use of birch AIT for treatment of PFS has been less successful (reviewed in Incorvaia et al104). Two randomized, placebo-controlled trials of birch AIT for PFS have been conducted, one for hazelnut and one for soy.105, 106 Neither trial demonstrated a statistically significant improvement in symptoms by double-blind placebo-controlled food challenge. In recent years, new high-quality standardized AIT products for birch pollen allergy have been developed, which could impact efficacy for PFS.25, 100 As new AIT products for birch pollen allergen are developed, the impact on PFS should be considered as a trial endpoint.
1.7 Clinical relevance of minor birch pollen allergens
Bet v 2 and Bet v 4 are members of the profilin and polcalcin protein families, respectively.13 Extracts of alder, hornbeam, hazel, and oak are IgE cross-reactive toward Bet v 2.5 IgE cross-reactivity toward birch pollen allergens and non-tree allergens such as grass, mugwort, latex, and/or olive tree has also been identified.36, 107 This broad cross-reactivity is primarily due to homology among allergens of the profilin and polcalcin protein families,11, 108 and the clinical relevance of sensitization to these proteins is questionable. A study of 200 pollen-allergic patients in Italy found that of the 50 patients reactive to date palm profilin and/or polcalcin, most of the patients only experienced symptoms during the grass pollen season, indicating the clinical relevance of IgE against these proteins in relation to birch pollen is limited.109 IgE against Bet v 2 has a high level of cross-reactivity with the grass pollen profilin Phl p 12,36 and a study of the clinical relevance of profilin proteins indicated that sensitization to Bet v 2 in the absence of Bet v 1 sensitization was not associated with allergy symptoms during birch pollen season, but symptoms were present during grass pollen season.36 Thus, Bet v 2 sensitization is primarily reflective of profilin cross-reactivity, and sensitization to Bet v 2 may be considered a marker of polysensitization, but is of no clinical relevance in allergic rhinitis.22
2 CONCLUSIONS
Birch pollen allergy is a major source of allergic rhinitis in Europe, and symptoms extend beyond the birch pollen season due to the extensive IgE cross-reactivity and varying pollen seasons within the birch homologous group of trees. In addition, patients with birch pollen allergy also commonly suffer from PFS, which may add to the burden on HRQoL. Thus, birch pollen allergy has a substantial clinical impact on birch-sensitive patients.
ACKNOWLEDGMENTS
Medical writing and editorial assistance were provided by Erin P. Scott, PhD, of Scott Medical Communications, LLC. This assistance was funded by ALK-Abelló, Hørsholm, Denmark.
AUTHOR CONTRIBUTIONS
All authors contributed to the critical review of this manuscript and provided approval to submit.
CONFLICTS OF INTEREST
T Biedermann has served as a consultant or speaker and received honorarium from ALK-Abelló, Mylan, and Novartis. L. Winther has served as a consultant and received honorarium from ALK-Abelló. P. Panzner has served as an advisory board member or speaker for ALK, Stallergenes, AstraZeneca, and Novartis. S.J. Till has served as a consultant or speaker and received honorarium from ALK-Abelló. A. Knulst has served as consultant for ALK-Abelló and received sponsoring for research projects from ALK-Abelló and Thermo Fisher. E. Valovirta has served as an advisory board member for ALK and AstraZeneca and received travel grants and honoraria from Mylan, Orion Pharma, AllergoPharma, ALK, and AstraZeneca.




