Lipid Transfer Protein allergy in the United Kingdom: Characterization and comparison with a matched Italian cohort
Abstract
Background
Although pollen-related food allergy occurs in all European populations, lipid transfer protein (LTP) allergy is considered to manifest mainly in Mediterranean countries. We aimed to characterize adults presenting with LTP allergy in a northern European country.
Method
The clinical history and sensitization patterns of subjects born and residing in the United Kingdom (UK), with a prior diagnosis of LTP allergy and sensitization to the peach LTP allergen Pru p 3, were compared to UK subjects with pollen food syndrome (PFS). The sensitization patterns were also evaluated against a matched cohort of Italian subjects diagnosed with LTP allergy.
Results
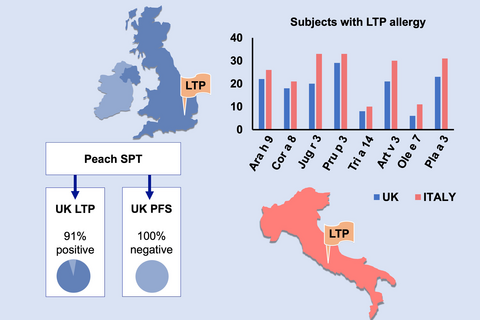
None of the 15 UK PFS subjects had a positive SPT to LTP-enriched peach reagent, compared to 91% of the 35 UK LTP subjects. The UK LTP cohort were also more likely to have positive skin prick tests to cabbage, lettuce and mustard and sensitization to the LTP allergens in peach, walnut, mugwort and plane tree These sensitization patterns to individual allergens were not significantly different to those obtained from the Italian LTP subjects, with significant correlations between Pru p 3 and the LTP allergens in peanuts, walnuts, plane tree and mugwort in both groups.
Conclusion
Native UK subjects with LTP allergy are not dissimilar to those with LTP allergy in southern Europe. Testing to LTP-enriched peach SPT reagent and/or LTP allergens in peach, walnut, mugwort and plane tree may enhance diagnostic accuracy.
Graphical Abstract
UK-born adults can develop Lipid Transfer Protein (LTP) allergy. Allergen sensitisation patterns in UK LTP allergic subjects are not significantly different to matched Italians with LTP allergy. Skin prick testing (SPT) with peach reagent is a useful diagnostic tool; the majority of UK LTP allergic subjects tested positive to peach, whereas none of those with Pollen Food Syndrome (PFS) did so.
1 INTRODUCTION
Allergy to fruits and vegetables most often presents in adults; a retrospective study on 2.7 million patients in the United States determined that the prevalence of allergy to fruits and vegetables to be 0.7%.1 In the UK, a survey of 3500 adults determined the most common food reported to provoke symptoms was noncitrus fruits (4.7%), with vegetables affecting 3.3%.2 In northern Europe, the most frequent manifestation is pollen food syndrome (PFS), presenting typically as mild oropharyngeal symptoms to raw fruits and vegetables, triggered by cross-reactions between antibodies to pollen and homologous plant food allergens.2-4 In southern Europe, in addition to PFS, another more severe manifestation of cross-reactive plant food allergy occurs, involving both raw and cooked foods but not clearly linked to pollen sensitization. Lipid transfer protein (LTP) allergy involves sensitization to LTP proteins, which are stable to heat and digestion and abundant in plant foods.5, 6 The peach LTP allergen Pru p 3 is a prototypic marker of LTP sensitization, with many of those sensitized also having clinical reactions on exposure to peaches, although many other foods can also provoke symptoms.7, 8 Although LTP allergy is not widely recognized in northern Europe, we conducted a pilot investigation of LTP sensitization in our UK clinic population using Pru p 3 as a surrogate marker of IgE sensitization.9 We found that sensitization to Pru p 3 was a feature of UK subjects experiencing severe reactions to fruits and vegetables, often in the absence of reported reactions to peaches. We hypothesized that in a group of patients who present with systemic allergic reactions and in whom there is evidence of LTP sensitization, as defined by a positive test to Pru p 3, there is a clear clinical and immunological profile compared to a group of patients with classical PFS, the most prevalent form of food allergy in the United Kingdom (UK).4 The aim of the study was to determine the main food and pollen sensitizations, suspected trigger foods and quality of life in UK patients with diagnosed LTP allergy, compared to UK subjects with classical PFS. We also aimed to evaluate the sensitization patterns of UK subjects with LTP allergy to an age- and sex-matched group of Italian (IT) subjects with LTP allergy, to understand whether there are key geographical differences in sensitization patterns in those with LTP allergy.
2 METHODS
This observational controlled cohort study was undertaken at the Royal Brompton & Harefield NHS Foundation Trust (RBHT) and Guy's and St Thomas’ NHS Foundation Trust (GSTFT), both located in London in the UK. The study received ethical approval from Portsmouth Research Ethics Committee and was also approved and sponsored by RBHT and GSTFT.
2.1 Participants
Participants aged 18 years and over were recruited between 2013 and 2016 and gave written informed consent to take part. To minimize the confounding effects of LTP sensitization arising outside northern Europe, the LTP case group were all born in the UK without extended periods of residence in southern Europe. They were recruited following a confirmed medical diagnosis of LTP allergy made in either the RBHT or GSTFT specialist allergy clinics, which are tertiary referral centres for food allergy. The diagnosis was based on a history of severe reactions, including severe orbital or oropharyngeal swelling, difficulty in breathing, tachycardia, collapse and anaphylaxis, to a suspected plant food-derived trigger(s) (see also Appendix S1), positive skin prick tests and specific IgE blood tests to the suspected food (s) involved and a Pru p 3 level > 0.35 kUA/L (ImmunoCAP assay, Thermo Fisher Scientific). Patients in whom the index reaction(s) was attributed to a primary nut allergy were excluded, such as those who had a positive Pru p 3 ImmunoCap, but reported symptoms only to nuts and had sensitization to other primary nut allergens. However, those who had reactions to several foods in addition to nuts were included. The control group were UK subjects diagnosed with PFS, using a validated PFS diagnostic questionnaire.2 A third group of age- and sex-matched Italian (IT) subjects from Rome, Italy, with a diagnosis of LTP food allergy, based on a positive specific IgE to Pru p 3 by ImmunoCap ISAC were used as a reference comparison group for the blood test results from the UK LTP group.
2.2 Interventions
2.2.1 Questionnaires
All subjects self-completed a standardized nonvalidated questionnaire on their clinical symptom history, suspected foods, the presence or absence of other allergic conditions and the use of adrenaline and other medications. On enrolment, they also completed a validated quality of life questionnaire, the Food Allergy Quality of Life Questionnaire—Adult Form (FAQLQ-AF).10
2.2.2 Skin Prick Tests
All subjects underwent Skin Prick Tests (SPT) to a panel of 40 aeroallergen and food extracts (either from ALK Abelló, Horsholm, Denmark or from Stallergenes, Antony, France), fresh foods and positive and negative control solutions (histamine hydrochloride 10 mg/mL and diluent) (see repository). Testing with the same variety of fresh food was undertaken using the prick to prick test method; the intact food was pierced through the peel or skin with a sterile lancet (ALK Abelló) and then used to prick the skin of the subject. All SPT were performed by the same operative, using standardized techniques according to international guidelines.11, 12 The test was considered positive if the size of any resulting wheal was ≥ 3 mm greater than the negative control.12 The foods tested were chosen based on known LTP triggers in other populations, and UK pilot data on reported foods in LTP subjects.13-16 Fresh foods were used for some foods tested where it was considered that they would provide a better result based on published research.17, 18 Peach extract (ALK Abelló) was chosen prospectively as a marker for LTP sensitization and birch pollen as for a marker for PFS related to PR-10 proteins.2, 19
2.2.3 Serum analysis
A semi-quantitative allergen microarray assay was used to determine the individual participant's specific IgE sensitization to 112 allergen components, measured using the ImmunoCAP 112 ISAC platform according to the manufacturer's instructions (Thermo Fisher Scientific). Specific IgE values were expressed in ISAC standard units (ISU), with values of 0.3 ISU or greater considered positive, with values grouped into established ranges (<0.3 ISU, not detectable; ≥0.3 to < 1 ISU, low; ≥1 to < 15, moderate; and ≥ 15, very high).
2.3 Statistical analysis
This was an exploratory study, so no formal sample size calculation was undertaken as there was no primary outcome of interest and the size of the sample was chosen based on feasibility constraints. As a result, analysis presented here is interpreted as hypothesis generating. As a tool for exploring possible differences between data sets, chi-squared or Mann-Whitney tests were used but adjustments were not made for multiple comparisons. The Pearson correlation was used to assess the association between two linear variables.
3 RESULTS
3.1 Subjects and clinical history
The case group consisted of 35 UK-born adults with previously diagnosed LTP allergy, and a Pru p 3 IgE level > 0.35 kUA/L (mean 16.8 U/mL, range 0.90-66.4 IU/mL). The control group comprised 15 UK subjects with PFS. Although the groups were not age or sex matched, there were no age or gender differences between them (Table 1). There were no differences in atopic history, with childhood eczema, asthma and allergic rhinitis commonly reported (Table 1). The majority of the LTP and all of the PFS participants reported allergic rhinitis, with the PFS group reporting their main season to be in the Springtime (P = 0.017 Pearson chi-square) (Table 1).
| Variable | LTP (%) n = 35 | PFS (%) n = 15 | Value | Sig |
|---|---|---|---|---|
| Demographic details | ||||
| Mean age (range, 95% CI) | 38 (18-71, 32-43) | 34 (18-66, 26-40) | 23.016 | 0.732 |
| Median age | 33 | 30 | ||
| Variance | 206 | 171 | ||
| Std deviation | 14.385 | 13.083 | ||
| Female | 27 (77) | 9 (60) | 1.531 | 0.216 |
| Childhood history of atopy | ||||
| Eczema | 15 (42) | 5 (33) | 0.308 | 0.579 |
| Food allergy | 9 (26) | 3 (20) | 0.188 | 0.665 |
| Asthma | 23 (64) | 6 (40) | 3.768 | 0.152 |
| Hay fever | 20 (56) | 11 (73) | 1.92 | 0.383 |
| Current atopic conditions | ||||
| Asthma | 18 (50) | 4 (27) | 4.196 | 0.123 |
| Eczema | 14 (39) | 6 (27) | 2.912 | 0.233 |
| Hay fever | 29 (81) | 15 (100) | 3.381 | 0.184 |
| Season of allergic rhinitis | ||||
| Spring only | 6 (18) | 8 (53) | 5.619 | 0.017 |
| Summer only | 3 (9) | 1 (7) | 0.098 | 0.754 |
| Spring and Summer | 11 (31) | 4 (27) | 0.290 | 0.597 |
| All year | 10 (28) | 2 (13) | 1.228 | 0.288 |
| Not sure | 2 (6) | 0 (0) | 0.867 | 0.352 |
| Type of food provoking reactions | ||||
| Raw foods only | 9 (26) | 14 (93) | 18.682 | 0.000 |
| Raw and cooked food | 6 (18) | 1 (7) | 1.025 | 0.311 |
| Cooked food only | 8 (23) | 0 | 4.218 | 0.040 |
| Don't know | 11 (31) | 0 | 6.258 | 0.012 |
| How soon do reactions occur after eating? | ||||
| Touching lips | 2 (6) | 6 (40) | 0.869 | 0.003 |
| Biting and chewing | 2 (6) | 6 (40) | 8.868 | 0.003 |
| Within 5 min | 9 (26) | 10 (67) | 7.084 | 0.008 |
| Within 15 min | 11 (31) | 5 (33) | 0.005 | 0.946 |
| Within 1 h | 13 (37) | 1 (7) | 5.083 | 0.024 |
| Within 3 h | 2 (6) | 0 | 0.92 | 0.338 |
| Within 6 h | 1 (3) | 0 | 0.45 | 0.542 |
| More than 6 h | 1 (3) | 0 | 0.45 | 0.542 |
| Time to symptom resolution | ||||
| Up to 1 h | 6 (18) | 9 (60) | 9.184 | 0.002 |
| Up to 4 h | 15 (43) | 5 (33) | 0.681 | 0.416 |
| Up to 12 h | 6 (18) | 1 (7) | 0.957 | 0.328 |
| 24 h | 8 (23) | 1 (7) | 1.865 | 0.172 |
| Co-factor involvement in reactions | ||||
| Any co-factor | 25 (71) | 2 (13) | 14.267 | 0.000 |
| Exercising | 14 (40) | 2 (13) | 3.87 | 0.160 |
| Any exertion | 20(57) | 2 (13) | 4.778 | 0.029 |
| Alcohol | 13 (37) | 1 (7) | 5.251 | 0.072 |
| Aspirin/NSAID | 3 (9) | 0 (0) | 1.978 | 0.372 |
| Unwell | 3 (9) | 1 (7) | 0.081 | 0.960 |
Both groups reported similar food triggers (tree nuts, peanuts, apples, stone fruits, tomatoes) (Appendix S2); however, 93% of the PFS group reacted only to raw foods compared to 23% of the UK LTP group (P = 0.000 chi-square), who also described reactions involving composite meals such as pizza and curry (Appendix S2). There were also differences in the speed of onset of symptoms; a greater number of the UK PFS group had reactions on the food touching the lips or when biting or chewing (P = 0.003 Pearson chi-square), and they were also significantly more likely to recover within an hour than the UK LTP group (P = 0.002; Table 1). As expected, given that initial diagnosis of LTP allergy incorporated assessment of reaction severity, this group was characterized by more severe symptoms including oral and facial oedema, throat narrowing/closure, difficulty in breathing and wheeze/chest tightness compared to the PFS group (Appendix S1).
Reactions due to any co-factor such as exercise, alcohol and nonsteroidal anti-inflammatory drugs were reported by 71% of those with LTP allergy compared to 13% of those with PFS (P = 0.000 Pearson chi-square; Table 1). As might be expected, the majority (86%) of the LTP group had been prescribed and carried an adrenalin auto-injector compared to 0% in the PFS group and had a significantly greater number of emergency visits to hospital (Appendix S3). Results from the Quality of Life Questionnaire indicated significant differences between the LTP and PFS groups, especially in the domains for Buying Food, Eating Out, Anxiety and Interaction (Appendix S4).
3.2 Skin prick tests
One subject in the LTP group declined SPT; thus, results are presented for 34 UK LTP subjects and 15 UK PFS subjects. A significantly greater percentage of the UK LTP subjects had a positive SPT to plane tree (P = 0.04), mugwort (0.009) and parietaria (0.001) (Pearson chi-square), and also a significantly greater median wheal size (Mann-Whitney U) (Table 2). None of the UK PFS group had a positive SPT to peach reagent, compared to 91% of the UK LTP group (P = 0.000 Pearson R), whereas 10/15 (66%) of the UK PFS group were sensitized to fresh peach (see Table 2). The UK LTP group were also significantly more likely to have a positive SPT to cabbage, lettuce, mustard, raspberry, walnut, barley and sesame seed (P = 0.000 Pearson chi-square) (see Table 2). Median SPT wheal size was also significantly greater for these same foods in the LTP cohort (Mann-Whitney U), with peach reagent having the overall largest median wheal size (see Table 2). There was no correlation between SPT wheal sizes to peach reagent and titres of Pru p 3-specific IgE ImmunoCap on entry to the study (Pearson r = 0.0315, P = 0.857). The SPT results for peach reagent in the LTP group were strongly correlated with those obtained from fresh peach (r = 0.67, P = 0.000), walnut (r = 0.56, P = 0.000), peanut (r = 0.61, P = 0.000), tomato (r = 0.54, P = 0.001), lupin (r = 0.53, P = 0.001), grape (r = 0.51, P = 0.002) and almond (r = 0.51, P = 0.002) (Table 2). When comparing the SPT results with reported reactions to foods, carrots, celery, grapes, raspberry and mustard gave the best NPV, and tree nuts, peanuts, apples and stone fruit (apricots, peaches, plums) the best PPV in both groups (Appendix S5).
| Median SPT wheal size (mm) | Positive SPT (%) | Correlation of SPT diameter vs peach reagent in LTP subjects | ||||||
|---|---|---|---|---|---|---|---|---|
| LTP n = 34 | PFS n = 15 | Sig | LTP | PFS | Sig | Pearson r value | Sig | |
| Foods | ||||||||
| Peach reagent | 6 | 0 | 0.000 | 32 (91) | 0 (0) | 0.000 | 1 | N/A |
| Cabbage | 4.5 | 0 | 0.000 | 29 (83) | 2 (13) | 0.000 | 0.398 | 0.020 |
| Lettuce | 4 | 0 | 0.000 | 25 (71) | 2 (13) | 0.000 | 0.379 | 0.027 |
| Mustard | 5.25 | 0 | 0.000 | 28 (80) | 4 (27) | 0.000 | 0.464 | 0.006 |
| Raspberry | 6.25 | 3 | 0.000 | 33 (94) | 8 (53) | 0.001 | 0.387 | 0.024 |
| Walnut | 5 | 2 | 0.000 | 29 (83) | 5 (33) | 0.001 | 0.569 | 0.000 |
| Barley | 4 | 0 | 0.000 | 27 (77) | 4 (27) | 0.001 | 0.394 | 0.021 |
| Sesame seed | 2.75 | 0 | 0.001 | 17 (60) | 0 (0) | 0.001 | 0.387 | 0.024 |
| Peanut | 4.75 | 3.5 | 0.002 | 30 (86) | 7 (47) | 0.016 | 0.619 | 0.000 |
| Tomato | 4.5 | 2.5 | 0.002 | 27 (77) | 6 (40) | 0.023 | 0.543 | 0.001 |
| Banana | 3.5 | 0 | 0.003 | 20 (57) | 3 (20) | 0.012 | 0.318 | 0.066 |
| Strawberry | 4 | 6.5 | 0.002 | 27 (77) | 15 (100) | 0.058 | 0.221 | 0.208 |
| Lupin | 3 | 0 | 0.003 | 21 (60) | 3 (20) | 0.012 | 0.531 | 0.001 |
| Sunflower seed | 3.5 | 0 | 0.007 | 21 (60) | 3 (20) | 0.012 | 0.496 | 0.003 |
| Grape | 5 | 3.5 | 0.009 | 30 (86) | 8 (53) | 0.007 | 0.511 | 0.002 |
| Cashew | 1.75 | 0 | 0.008 | 10 (29) | 0 (0) | 0.019 | 0.127 | 0.475 |
| Pistachio | 1.75 | 0 | 0.007 | 13 (37) | 1 (7) | 0.024 | 0.055 | 0.755 |
| Macadamia | 3.5 | 0 | 0.030 | 24 (69) | 6 (40) | 0.070 | 0.296 | 0.089 |
| Orange | 3.5 | 2 | 0.011 | 26 (74) | 5 (33) | 0.008 | 0.387 | 0.024 |
| Celery salt | 3 | 0 | 0.052 | 20 (57) | 7 (47) | 0.551 | 0.326 | 0.060 |
| Celery | 3.5 | 2.5 | 0.074 | 25 (71) | 7 (47) | 0.109 | 0.317 | 0.068 |
| Candied orange | 0 | 0 | 0.102 | 3 (9) | 0 (0) | 0.235 | 0.026 | 0.885 |
| Wheat | 3.5 | 2 | 0.170 | 21 (60) | 5 (33) | 0.066 | 0.148 | 0.403 |
| Hazelnut | 4.75 | 4.5 | 0.218 | 28 (80) | 11 (73) | 0.638 | 0.221 | 0.210 |
| Brazil nut | 0 | 0 | 0.021 | 6 (17) | 0 (0) | 0.820 | 0.056 | 0.753 |
| Apple | 3.5 | 4 | 0.281 | 23 (66) | 11 (73) | 0.563 | 0.253 | 0.149 |
| Carrot | 3 | 4 | 0.524 | 21 (60) | 9 (60) | 0.907 | 0.278 | 0.111 |
| Kiwi | 5 | 4.5 | 0.922 | 29 (83) | 14 (93) | 0.311 | 0.297 | 0.088 |
| Maize | 3 | 3.5 | 0.799 | 22 (63) | 8 (53) | 0.451 | 0.121 | 0.494 |
| Fresh peach | 6 | 6 | 0.801 | 31 (88) | 10 (66) | 0.33 | 0.670 | 0.000 |
| Soy | 3.25 | 3.5 | 0.894 | 18 (51) | 7 (47) | 0.98 | 0.455 | 0.006 |
| Almond | 5 | 4.5 | 0.930 | 29 (83) | 12 (80) | 0.644 | 0.515 | 0.002 |
| Aeroallergens | ||||||||
| Parietaria | 2.5 | 0 | 0.002 | 16 (46) | 0 (0) | 0.001 | 0.277 | 0.113 |
| Plane | 5 | 2.5 | 0.032 | 26 (74) | 8 (53) | 0.04 | 0.334 | 0.053 |
| Mugwort | 3 | 0 | 0.014 | 18 (46) | 2 (13) | 0.009 | 0.247 | 0.159 |
| Silver birch | 4 | 6 | 0.064 | 23 (66) | 13 (87) | 0.165 | 0.042 | 0.813 |
| Latex | 0 | 0 | 0.79 | 6 (17) | 1 (7) | 0.311 | 0.185 | 0.294 |
| Ash | 3.25 | 4.5 | 0.486 | 20 (57) | 8 (53) | 0.72 | 0.040 | 0.824 |
| Timothy grass | 7.25 | 8 | 0.753 | 30 (86) | 13 (87) | 0.899 | 0.298 | 0.087 |
3.3 Microarray
The ISAC results showed clear patterns of sensitization. Tests to the primary allergens in peanuts (Ara h 1, Ara h 2, Ara h 3, Ara h 6), tree nuts (Ber e 1, Jug r 1, Cor a 9, Ana o 2) and sesame (Ses i 1) were seldom positive in both UK groups (Appendix S6). As might be expected, 94% of the UK LTP cohort had a positive test to one or more LTP allergens compared to 7% of the PFS UK group (Table 3). Whilst both groups were sensitized to PR10 allergens, a significantly greater number of PFS participants had a positive test to Cor a 1 (hazelnut), Pru p 1 (peach), Mal d 1 (apple) and Ara h 8 (peanut) (Table 3). Results for aeroallergens show significant differences in sensitization; a greater percentage of the UK LTP group had a positive test to the LTP allergens to mugwort (Art v 3) and plane tree (Pla a 3) (P < 0.001; Table 3), whereas the UK PFS groups were more likely to be sensitized to the PR10 allergens in trees including alder (Aln g 1), hazel (Cor a 1.0101) (P < 0.001) and to a lesser extent Bet v 1 (P = 0.007) (Table 3). There were no differences in sensitization to grass pollen allergens. Evaluation of median levels of ISU for LTP and PR10 food and aeroallergens showed few significant differences between the UK LTP and UK PFS Groups. As might be expected, the LTP group had greater median levels of the LTP allergens in hazelnuts, walnuts, peanuts and plane tree, and the UK PFS group higher levels of PR10 allergens in kiwifruit, alder and birch tree pollen (Table 3).
| Median ISAC test result (ISU) | Positive ISAC (%) | |||||
|---|---|---|---|---|---|---|
| LTP n = 35 | PFS n = 15 | Sig | LTP | PFS | Sig | |
| Foods | ||||||
| rAra h 9 | 1.2 | 0 | 0.031 | 22 (63) | 1 (7) | 0.000 |
| rCor a 8 | 1 | 0 | 0.007 | 18 (51) | 0 | 0.001 |
| nJug r 3 | 2.9 | 0 | 0.014 | 30 (86) | 1 (7) | 0.000 |
| rPru p 3 | 1.6 | 0 | 0.012 | 29 (83) | 1 (7) | 0.000 |
| rTri a 14 | 0 | 0 | 0.790 | 8 (23) | 0 | 0.095 |
| rCor a 1.0401 | 0 | 5.4 | 0.163 | 13 (37) | 13 (87) | 0.001 |
| rMal d 1 | 0 | 11 | 0.085 | 13 (37) | 13 (87) | 0.001 |
| rPru p 1 | 0 | 3.4 | 0.722 | 8 (23) | 13 (87) | 0.001 |
| rGly m 4 | 0 | 0 | 0.761 | 4 (11) | 7 (47) | 0.006 |
| rAra h 8 | 0 | 1.5 | 0.317 | 5 (14) | 9 (60) | 0.001 |
| rAct d 8 | 0 | 0 | 0.016 | 3 (9) | 5 (33) | 0.028 |
| rApi g 1 | 0 | 0 | 0.438 | 3 (9) | 4 (27) | 0.091 |
| Aeroallergens | ||||||
| Cup a 1 | 0 | 0 | 0.858 | 7 (20) | 3 (20) | 1.000 |
| nCyn d 1 | 2.1 | 0.8 | 0.351 | 21 (60) | 9 (60) | 1.000 |
| rPhl p 1 | 8.4 | 9.4 | 0.366 | 25 (71) | 12 (80) | 0.728 |
| rPhl p2 | 0 | 0 | 0.883 | 15 (43) | 5 (33) | 0.274 |
| nPhl p 4 | 2 | 3 | 0.404 | 21 (60) | 9 (60) | 1.000 |
| rPhl p 5 | 4.7 | 17 | 0.686 | 23 (66) | 11 (73) | 0.746 |
| rPhl p 6 | 1 | 1.8 | 0.615 | 18 (51) | 8 (53) | 0.359 |
| rPhl p 11 | 0 | 0 | 0.413 | 5 (14) | 5 (33) | 0.123 |
| rBet v 1 | 0 | 25 | 0.008 | 16 (46) | 13 (87) | 0.007 |
| rPar j 2 | 0 | 0 | 0.778 | 3 (9) | 1 (7) | 0.820 |
| Art v 1 | 0 | 0 | 0.603 | 1 (3) | 1 (7) | 0.529 |
| nArt v 3 | 1.3 | 0 | 0.066 | 21 (60) | 0 | 0.000 |
| nOle e 1 | 0 | 0 | 0.479 | 5 (14) | 5 (33) | 0.390 |
| nOle e 7 | 0 | 0 | 0.197 | 6 (17) | 0 | 0.087 |
| nOle e 9 | 0 | 0 | 0.549 | 0 | 0 | 0.549 |
| Pla a 1 | 0 | 0 | 0.145 | 0 (0) | 1 (7) | 0.123 |
| Pla a 2 | 0 | 0 | 0.075 | 7 (20) | 1 (7) | 0.381 |
| rPla a 3 | 2.4 | 0 | 0.010 | 23 (66) | 0 | 0.000 |
| rAln g 1 | 0 | 3.4 | 0.041 | 6 (17) | 13 (87) | 0.000 |
| r Cor a 1.0101 | 0 | 1.8 | 0.267 | 6 (17) | 12 (60) | 0.000 |
| rBet v 2 | 0 | 0 | 0.327 | 8 (23) | 3 (20) | 0.823 |
| rPhl p 12 | 0 | 0 | 0.345 | 8 (23) | 2 (13) | 0.440 |
When the Pru p 3 ISU level on ISAC in the UK LTP cohort was compared to the ImmunoCap Pru p 3 level on entry to the study, the former had a sensitivity of 82% (95%CI—65%-93%, +ve LR 0.82). In contrast, the Peach SPT reagent had a sensitivity of 91% (95% CI—76%-98%, +ve LR 0.91). There was no correlation between symptom severity and the level of Pru p 3 ImmunoCap on entry to the study or level of Pru p 3 ISU in the ISAC, or between symptom severity and number of LTP sensitizations. One LTP subject was not sensitized to any LTP allergens in the microarray; this subject had a Pru p 3 level of 1.37 on entry to the study but a negative SPT to peach reagent.
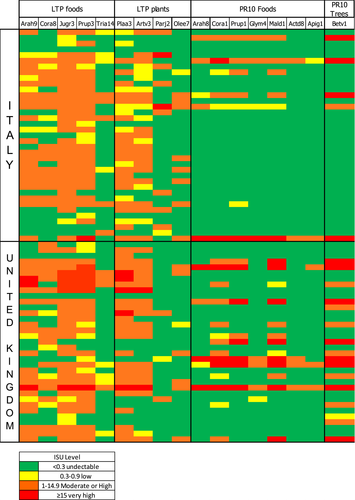
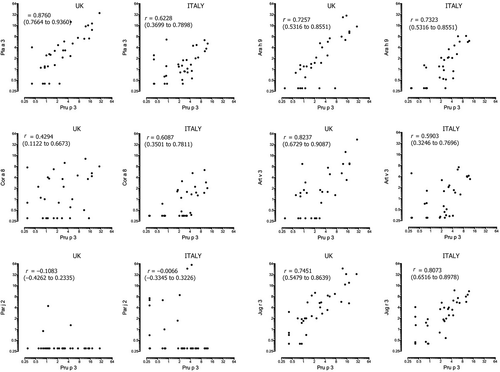
When the UK LTP ISAC microarray results were compared with that from age- and sex-matched Italian subjects with LTP allergy (IT LTP group), the number of subjects in each group sensitized to any individual food allergen, including LTP and PR10 allergens, was comparable (Table 4 and Figure 1). However, sensitization to the grass pollen allergens Phl p 1, Phl p 2, Phl p 4, Phl p 5, Phl p 6 and to a lesser extent to Bet v 1 was significantly more prevalent in the UK LTP group, and the IT LTP cohort were more likely to be sensitized to Cup a 1 (Cypress) (see Table 3). There were no differences for other tree or weed allergens including Pla a 1, Pla a 2 and Art v 1 (Table 3). The median ISU level was significantly greater for Phl p 1, Phl p 2, Phl p 4, Phl p 5, Phl p 6 and Bet v 1 in the UK LTP group, with the IT LTP group having greater median ISU levels of Pla a 1, Pla a 3, Cup a 1, Par j 2 and Cyn d 1 (Mann-Whitney U) (Table 4). When the mean ISU level of the LTP allergens in the ISAC was correlated against the mean level of ISU for Pru p 3 in the ISAC, there were highly significant correlations between Pru p 3 and Pla a 3, Art v 3, Ara h 9, Jug r 3, for both UK and IT LTP groups with the IT LTP group additionally having a significant correlation between Pru p 3 and Cor a 8 (Figure 2). In both groups, there was a poor correlation between the mean ISU level of Pru p 3 and the parietaria LTP allergen Par j 2. In the UK LTP group, in addition to Pru p 3, the mean ISU level of Jug r 3 also was strongly correlated to the ISU level of other LTP allergens on the ISAC, whereas Ara h 9 and Cor a 8 had few significant correlations to other LTP allergens and Tri a 14 had none (Appendix S7).
| Positive ISAC (%) | Median ISAC test result (ISU) | |||||
|---|---|---|---|---|---|---|
| UK LTP n = 35 (%) | IT LTP n = 37 (%) | Sig | UK LTP n = 35 | IT LTP n = 37 | Sig | |
| Foods | ||||||
| Ara h 9 | 22 (63) | 26 (70) | 0.505 | 1.2 | 0.94 | 0.481 |
| Cor a 8 | 18 (51) | 21 (57) | 0.555 | 1 | 0.25 | 0.162 |
| Jug r 3 | 30 (86) | 33 (89) | 0.656 | 2.9 | 2.31 | 0.254 |
| Pru p 3 | 29 (83) | 33 (89) | 0.437 | 1.6 | 2.4 | 0.951 |
| Tri a 14 | 8 (23) | 10 (27) | 0.920 | 0 | 0 | 0.700 |
| Cor a 1.0401 | 13 (37) | 6 (16) | 0.044 | 0 | 0 | 0.913 |
| Mal d 1 | 13 (37) | 6 (16) | 0.044 | 0 | 0 | 0.040 |
| Pru p 1 | 8 (23) | 7 (19) | 0.523 | 0 | 0 | 0.670 |
| Gly m 4 | 4 (11) | 4 (11) | 0.934 | 0 | 0 | 0.934 |
| Ara h 8 | 5 (14) | 5 (14) | 0.925 | 0 | 0 | 0.903 |
| Act d 8 | 3 (9) | 2 (5) | 0.957 | 0 | 0 | 0.655 |
| Api g 1 | 3 (9) | 2 (5) | 0.957 | 0 | 0 | 0.600 |
| Aeroallergens | ||||||
| Cup a 1 | 7 (20) | 23 (62) | 0.000 | 0 | 1.98 | 0.001 |
| Cyn d 1 | 21 (60) | 12 (32) | 0.019 | 0.8 | 0 | 0.016 |
| Phl p 1 | 25 (71) | 15 (41) | 0.008 | 9.4 | 0 | 0.001 |
| Phl p2 | 15 (43) | 0 (0) | 0.000 | 0 | 0 | 0.000 |
| Phl p 4 | 21 (60) | 5 (14) | 0.000 | 3 | 0 | 0.000 |
| Phl p 5 | 23 (66) | 9 (24) | 0.000 | 17 | 0 | 0.000 |
| Phl p 6 | 18 (51) | 7 (19) | 0.003 | 1.8 | 0 | 0.033 |
| Phl p 11 | 5 (14) | 2 (5) | 0.204 | 0 | 0 | 0.216 |
| Bet v 1 | 16 (46) | 6 (16) | 0.007 | 25 | 0 | 0.012 |
| Par j 2 | 3 (9) | 11 (30) | 0.230 | 0 | 0 | 0.018 |
| Art v 1 | 1 (3) | 1 (3) | 0.968 | 0 | 0 | 0.953 |
| Art v 3 | 21 (60) | 30 (81) | 0.049 | 0 | 0.62 | 0.806 |
| Ole e 1 | 5 (14) | 8 (22) | 0.378 | 0 | 0 | 0.704 |
| Ole e 7 | 6 (17) | 11 (30) | 0.209 | 0 | 0 | 0.437 |
| Ole e 9 | 4 (11) | 3 (8) | 0.635 | 0 | 0 | 0.547 |
| Pla a 1 | 0 (0) | 4 (11) | 0.045 | 0 | 0 | 0.047 |
| Pla a 2 | 7 (20) | 10 (27) | 0.478 | 0 | 0 | 0.658 |
| Pla a 3 | 23 (66) | 31 (84) | 0.165 | 0 | 1.08 | 0.041 |
| Aln g 1 | 6 (17) | 5 (14) | 0.669 | 3.4 | 0 | 0.646 |
| Cor a 1.0101 | 6 (17) | 7 (19) | 0.845 | 1.8 | 0 | 0.026 |
| Bet v 2 | 8 (23) | 3 (8) | 0.082 | 0 | 0 | 0.100 |
| Phl p 12 | 8 (23) | 3 (8) | 0.082 | 0 | 0 | 0.075 |
4 DISCUSSION
Our data are the first to indicate that LTP allergy is manifest in UK-born adults, with sensitization patterns to LTP allergens similar to those seen in matched Italian subjects. The geographical variation in plant food allergies is well reported, but our data add to published evidence that LTP allergy occurs in northern regions of Mediterranean countries and also other regions of Europe.20, 8, 21, 22 Recent studies include one on Austrian subjects with severe reactions to plant foods, who have been shown to be sensitized to Pru p 3, and a large data set has demonstrated that LTP sensitization is present in subjects living in Belgium.23, 24 Outside of Europe, data from China show that the most common sensitizing allergen in peanut allergy is Ara h 9, with a strong correlation between peanut, mugwort pollinosis and peach allergy.25
Although Pru p 3 is a common sensitizing allergen, not all those with LTP allergy will react to peaches.26, 27 Only four UK LTP subjects specifically mentioned peaches were a known trigger of reactions, although 18/35 did specify a stone fruit (peaches, nectarines, plums, cherries or apricots) was suspected (Appendix S2). A positive Pru p 3 specific IgE has been shown to be a marker of LTP allergy to tomatoes, orange and cabbage, although the primary reported food provoking reactions in LTP subjects may possibly dictate other food triggers.15, 28-30 The foods involved in the UK PFS and LTP groups appear to be similar. However, the PFS cohort mainly reported reactions only to raw foods, whereas UK LTP subjects reacted to a wide range of foods, including processed foods, reflecting the heat stable nature of LTP allergens compared to PR10 allergens.31, 32 Cooked or processed foods still contain LTP allergens; tomato paste, puree and canned tomatoes all have been shown to contain detectable levels of LTP allergens.33 The foods reported by the UK LTP cohort (nuts, apples, stone fruit, tomatoes and curry/spicy food) were similar to those reported by other studies to be the most common LTP trigger foods.34, 35 What is unusual is that around 25% of UK LTP subjects reported reactions only to cooked foods, which may reflect differences in local eating habits and food exposures, but also link to the potential difference in the primary sensitizing allergen in our UK cohort which is still unknown. Interestingly, banana and carrot were cited as trigger foods by the UK LTP cohort, but have been considered as safe foods by others.36
Asero and colleagues demonstrated that there is cross-reactivity between LTPs in both fruits and vegetables, frequently accompanied by more severe and systemic reactions than those manifest in PFS.37, 38 Our data demonstrated this, although differences in symptom severity would be expected, given the basis on which the groups were recruited; therefore, our severity data cannot be compared to other studies. Scala et al39 reported that subjects who reacted to >5 LTPs experienced a greater number of food-induced systemic reactions, which was thought to be due to the lower incidence of co-sensitization to other pan-allergens such as PR10. However, this finding was not replicated in our cohort possibly due to the way the UK LTP cohort was recruited. Also, the Italian study involved data from many more subjects (568), and despite the common sensitization patterns, there may be other differences between northern and southern European expressions of LTP allergy.
Our ISAC results clearly showed that there were no significant differences between the UK and IT subjects for sensitization to food allergens. Both groups were unlikely to be sensitized to other class 1 allergens except for Jug r 2, which was positive in two of the IT group and 10 of the UK group (Figure 2). The ISAC positivity of this natural allergen is affected by cross-reactive carbohydrate determinants (CCD) recognition in many cases, however only 1/2 of the IT subjects and 4/10 of the UK subjects were co-sensitized to MUXF3, the CCD on the ISAC, so this sensitization might be relevant.40 There was no difference in sensitization to mugwort and plane tree between the UK and IT LTP cohorts; however, the UK cohort had greater r values for the correlation between Pru p 3 and Art v 3 (r = 0.823), and Pru p 3 and Pla a 3 (r = 0.76) was higher than for the same parameters in the IT LTP cohort. Faber and colleagues only reported a modest r value of 0.48 between Art v 3 and Pru p 3, with much stronger correlations reported between Pru p 3 and other food LTP allergens such as Mal d 3 (0.91) and Cor a 8 (0.69) in their population from Belgium.24 However, other studies have demonstrated an association between these pollens, Pru p 3 and peach allergy.27, 41 Pla a 3 has been associated with severe reactions to foods and, together with Art v 3, is also linked to respiratory symptoms in LTP-allergic individuals.38, 41 Also, a correlation has been observed between sensitization to Pla a 3 and tree nut/peanut LTPs but in our cohort only walnut (Jug r 3) had a positive correlation with Pla a 3 (r = 0.71) (Appendix S1). Sanchez-Lopez and colleagues found Art v 3 could elicit rhinitis in sensitized patients, suggesting that a primary sensitization to Pru p 3 may lead to a respiratory allergy through cross-reactivity.42 Exposure to high levels of plane tree pollen in London could explain the high rate of Pla a 3 sensitization in the UK LTP group. Whilst this might suggest plane tree could be important in developing LTP allergy, it does not explain why none of the PFS group were sensitized to Pla a 3 and there was little sensitization to other plane tree allergens Pla a 1 and Pla a 2. Thus, Pla a 3 may only be positive in the LTP group due to cross-reactivity to Pru p 3.35 It has been postulated that Par j 2 is associated with a lower prevalence of severe food-induced reactions, due to a low cross-reactivity with food LTPs, Art v 3 and Pla a 3.38 Interestingly, although there was a higher level of SPT positivity to parietaria in the UK LTP group, sensitization to Par j 2 was rare. A high level of birch pollen sensitization has also been linked to a low prevalence of LTP allergy. However, 43% of the UK LTP subjects were also sensitized to Bet v 1 suggesting other factors might be involved in the pathogenesis of LTP allergy in the UK.
Goikoetxea and colleagues found peach SPT to be a sensitive technique for detecting sensitization to LTP and our findings concur with this.43 The large number of positive SPT in the LTP group was expected; the foods chosen were known LTP triggers and also strongly likely to cross-react meaning the positive predictive value is poor. Romano and colleagues showed that peanut sensitization was frequent among LTP-allergic patients but was only clinically significant in only about 50% of cases.26 Our data did reveal a modest correlation between peach SPT and positive SPT to peanut, lupin, walnut, almond, tomato and grape in the UK LTP cohort, many of which were cited as trigger foods. We speculate that these foods may have value as markers of LTP sensitization in UK subjects. A positive test to one of these foods, in those who either have a negative peach SPT or if peach SPT has not been tested, may be an indicator to consider component testing for LTP allergy.
Our data suggest Pru p 3 is the best marker for LTP sensitization in the UK, despite a low level of reported reactions to peaches in the UK LTP cohort. This concords with the findings from Mothes Luktsch et al23 who concluded that although apricots were a more likely trigger food in their Austrian LTP cohort, Pru p 3 was a good marker of LTP allergy. Both the UK and IT LTP cohorts included several subjects who had a negative ISAC test to Pru p 3, but universal sensitization to Pru p 3 is not always the case for those with LTP allergy.38 The UK LTP cohort had a range of levels of sensitization to Pru p 3, suggesting that the level may only be partially predictive of clinical allergy; this finding is supported by other investigators.35 One limitation of our study is that only those LTP allergens available on the ISAC were tested. Palacin and colleagues reported that although Pru p 3 had the highest recognition frequency, the LTP in apples, oranges, cabbage and mustard was also highly recognized, none of which are currently available on the ISAC array.44
Adults with a food allergy have a poorer quality of life, compared with those who have other chronic conditions such as diabetes,45 and recent data have shown that this is also true for those with PFS.46 Our data show that LTP allergy also significantly affects quality of life, possibly due to the number of potential food triggers and the link to co-factors making it difficult to predict whether a reaction to a particular food might occur. However, we accept that due to the study design, our LTP cohort all reported severe reactions and so might be more likely to have a poorer quality of life, whereas there is a spectrum of severity in LTP allergy reported in other studies.39 Our data also suggest that co-factors are an issue for UK subjects with LTP allergy; 40% of our cohort reported that their reactions to foods were associated with one or more co-factors.
The strength of our study was that the UK LTP cohort were all born in the UK and had not lived long periods out of the UK. In addition, they had all received their diagnosis from two of the study authors, using similar criteria and diagnostic tests, although no oral food challenges were performed. The UK PFS subjects were all diagnosed using a published diagnostic questionnaire validated against a standard diagnostic pathway including oral food challenge.4 However, the authors accept that the study design precludes the determination of the prevalence of LTP allergy in the UK. One weakness of the study is that diagnosis of LTP allergy was made on clinical history, positive specific IgE antibody tests to suspected foods and a positive specific IgE test to Pru p 3 ImmunoCap, rather than the gold standard of oral food challenge. However, oral food challenges were not undertaken because in many cases the precise ingredients provoking the reaction were not identified. High rates of cross-reactivity between LTP allergens can make the identification of a trigger food more difficult, or the trigger reported was a composite food which makes undertaking a standardized oral food challenge very difficult. Other studies on LTP allergy have often taken a cohort of patients who all have a primary allergy to the same food, facilitating the use of standardized oral food challenges.
In summary, we have shown that adults born and living in the UK can develop LTP allergy, with food triggers and co-factor involvement similar to those reported by other studies. The allergen sensitization patterns in the UK LTP subjects were not significantly different to those in a matched Italian cohort. Thus, LTP sensitization may be prevalent in countries previously considered as unlikely venues for this severe food allergy. The establishment of sensitization to LTP allergens in a wider range of countries has undoubtedly been partially driven by the advent of component-resolved diagnosis and multiplex testing, which has enabled analysis of large data sets, such as that undertaken in Belgium.24 Our data suggest that the diagnostic pathway for LTP allergy can be commenced by undertaking SPT with LTP-enriched peach reagent, a practice others have already shown to be effective.47 However, Tuppo et al48 demonstrated that LTP-enriched peach reagent contains other peach allergens such as Pru p 1, 2, 4 and 7. Thus, it might be expected that some of the UK PFS group would have had a positive test to the peach SPT reagent, especially since many had a positive test to the fresh peach, but this was not the case. Nevertheless, in order to confirm a diagnosis of suspected PFS in an individual with a positive SPT to peach reagent, it is important undertake CRD. Our data suggest that in a UK population, the key allergen to test for is Pru p 3, with other LTP allergens such as Pla a 3, Art v 3 and Jug r 3 also supporting the diagnosis. Our data indicate that the ImmunoCap test for Pru p 3 may be a best first line diagnostic test, since two subjects with a positive Pru p 3 ImmunoCap had no positive LTP on the ISAC. The geographical reach of LTP allergy appears to be growing, although it is possible that this is not a new phenomenon in the UK population, but is only now is being revealed through increased awareness and the use of component-resolved diagnosis.
ACKNOWLEDGMENTS
We would like to acknowledge Dr Victoria Cornelius for her very helpful statistical advice. This project was funded in part by a research grant awarded by “Friends of Guy's Hospital” charity.
AUTHOR CONTRIBUTIONS
IS, ST and LC designed and completed the UK study, and ES contributed the Italian patients. MS organized and oversaw serum sample analysis. IS wrote the manuscript which was reviewed and amended by ST, LC, ES and MS.