Comparative outcomes for over 100 deceased donor kidney transplants from SARS-CoV-2 positive donors: A single-center experience
Abstract
Emerging data support the safety of transplantation of extra-pulmonary organs from donors with SARS-CoV-2-detection. Our center offered kidney transplantation (KT) from deceased donors (DD) with SARS-CoV-2 with and without COVID-19 as a cause of death (CoV + COD and CoV+) to consenting candidates. No pre-emptive antiviral therapies were given. We retrospectively compared outcomes to contemporaneous DDKTs with negative SARS-CoV-2 testing (CoVneg). From February 1, 2021 to January 31, 2022, there were 220 adult KTs, including 115 (52%) from 35 CoV+ and 33 CoV + COD donors. Compared to CoVneg and CoV+, CoV + COD were more often DCD (100% vs. 40% and 46%, p < .01) with longer cold ischemia times (25.2 h vs. 22.9 h and 22.2 h, p = .02). At median follow-up of 5.7 months, recipients of CoV+, CoV + COD and CoVneg kidneys had similar rates of delayed graft function (10.3%, 21.8% and 21.9%, p = .16), rejection (5.1%, 0% and 8.5%, p = .07), graft failure (1.7%, 0% and 0%, p = .35), mortality (0.9%, 0% and 3.7%; p = .29), and COVID-19 diagnoses (13.6%, 7.1%, and 15.2%, p = .33). Though follow-up was shorter, CoV + COD was associated with lower but acceptable eGFR on multivariable analysis. KT from DDs at various stages of SARS-CoV-2 infection appears safe and successful. Extended follow-up is required to assess the impact of CoV + COD donors on longer term graft function.
Abbreviations
-
- CIT
-
- cold ischemia time
-
- COVID-19
-
- coronavirus disease 2019
-
- DBD
-
- donation after brainstem death
-
- DCD
-
- donation after cardiac death
-
- DGF
-
- delayed graft function
-
- KDPI
-
- kidney donor profile index
-
- KT
-
- kidney transplant
-
- OPO
-
- organ procurement organization
-
- OPTN
-
- Organ Procurement Transplant Network
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
1 INTRODUCTION
Since the start of the SARS-CoV-2 pandemic, death rates have increased significantly for all groups over age 15 and COVID-19 became the third leading cause of death.1 While this is sobering, organ donation is a potential silver lining to increased death rates from any cause, particularly for younger individuals with unexpected deaths and preserved organ function. With the high prevalence of SARS-CoV-2 infection and the ongoing organ shortage, it was inevitable that the transplant community would have to directly address the use of organs from donors with SARS-CoV-2 RNA detection and from those dying of COVID-related causes with preserved organ function.
As was predicted by the pathophysiology of RNA respiratory viruses, transplantation of lungs from such donors would prove to be prohibitive. However, the use of extra-pulmonary organs from donors infected with SARS-CoV-2 has been controversial. Guidance from the Organ Procurement and Transplant Network Ad Hoc Disease Transmission Advisory Committee (OPTN-DTAC) recommends that programs balance the risk for SARS-CoV-2 transmission, the possible effects on allograft quality, and the risk to the procurement teams with the recipient's risk for mortality and other complications on the waitlist.2 However, programs and organ procurement organizations (OPOs) must calculate these theoretical risks without additional guidance and may weigh the risk of transplantation too highly. Thus far, productive infection of extra-pulmonary organs remains unproven.3-5 And while acute kidney injury is described for about a third of those hospitalized for COVID-19, it is often associated with other risk factors for renal injury and most often recovers.6-8 Those with mild COVID-19 are even less likely to experience effects on kidney function.7
Due to caution at many levels, data for the use of organs from SARS-CoV-2 donors have grown slowly. Inadvertent transplantation from SARS-CoV-2 positive donors has led to devastating infection in lung recipients.9, 10 However, the use of extra-pulmonary organs from the same donors has resulted in no clinical signs of donor-derived SARS-CoV-2 and no reported ill- effects on allograft or patient outcomes.9, 11 Case reports, small case series and now recent summative OPTN data of liver, kidney and heart transplantation from donors with SARS-CoV-2 detection have been published with excellent recipient outcomes.12-25 While the OPTN data is supportive of the safe use of organs from donors with SARS-CoV-2 detection, donor and recipient details that may illustrate the likelihood of active infection or infection-related complications are lacking.25
We previously reported our first 10 kidney transplants from SARS-CoV-2 positive donors many of whom may not have had active SARS-CoV-2 infection at the time of donation.26 In the absence of alternative data to support a safety risk to our transplant candidates, we gradually loosened our restrictions for acceptance of kidneys from SARS-CoV-2 positive donors including those with evidence of active infection and those with death from COVID-related causes, organs that were often otherwise discarded during this time period. We aimed to report the safety of using these organs and the clinical outcomes compared to recipients of kidneys from CoVneg donors.
2 METHODS
This is a retrospective study of all single adult kidney transplant recipients of deceased donors performed at the Cleveland Clinic from February 1, 2021 to January 31, 2022. We collected deceased donor data including details of SARS-CoV-2 testing and infection and recipient clinical and laboratory baseline and follow-up data. CoV+ donors were defined as those testing positive for SARS-CoV-2 within 72 h of OPO evaluation without a COVID-related cause of death. Those with SARS-CoV-2 detected during the index hospitalization and a COVID-related cause of death were labeled CoV + COD. Resolved COVID-19 was defined as a history of confirmed COVID-19 with the resolution of symptoms and signs 21 to 90 days from the date of symptom onset. This retrospective study was approved by the Cleveland Clinic Institutional Review Board.
In early 2021, our adult kidney transplant program put forth a protocol to consider the transplantation of kidneys from donors testing positive for SARS-CoV-2. At all times we accepted donors with otherwise acceptable donor characteristics and standard measures of good organ function. Early, we aimed to avoid donors with signs of active SARS-CoV-2 infection, particularly those with severe COVID-19 or with COVID-related inflammatory syndromes. In August 2021, we accepted any donor outside of the first 2 weeks of symptomatic infection as best determined by OPO donor history, imaging findings, timing of first positive SARS-CoV-2 test, and SARS-CoV-2 PCR cycle threshold greater than 30. This allowed for the use of kidneys from those dying from recent COVID-related hypoxemic respiratory failure but with preserved kidney function. As of December 2021, our protocol allowed for the use of organs from any donor with favorable markers of kidney function regardless of the timing of SARS-CoV-2 infection. This included donors with markers of active acute SARS-CoV-2 infection.
There were no recipient selection criteria other than the willingness to accept such an organ. By November 2021, all adult transplant candidates at our institution were required to be fully vaccinated with at least two doses of mRNA vaccine (Pfizer-BioNTech's BNT162b2 or Moderna's mRNA-1273) or one dose of SARS-CoV-2 viral vector vaccine (Johnson and Johnson's Jannsen) as a condition for transplantation. All recipients consented to the use of organs from SARS-CoV-2 (COVID-19) positive donors at the time of organ offer. All recipients received our standard immunosuppression without regard to donor SARS-CoV-2 or COVID-19 status. This typically included induction with rabbit anti-thymocyte globulin (rATG), tacrolimus, mycophenolate mofetil (MMF), and prednisone. Recipients received no pre-emptive antiviral therapies, including direct-acting agents or passive antibody therapies. Recipients were monitored for signs of SARS-CoV-2 clinical infection and standard markers of allograft function. Patients with unexplained febrile or respiratory illness were evaluated with chest imaging and SARS-CoV-2 testing as indicated. Posttransplant COVID-19 diagnoses were also identified by any routine testing required at hospital admission or prior to procedures and by patient report of a positive test documented in the hospital chart on follow-up. Allograft rejection was defined by evidence of biopsy-proven acute cellular rejection or antibody-mediated rejection that led to directed therapies for these diagnoses. Biopsies were obtained by protocol when feasible or due to signs of allograft dysfunction.
2.1 Statistical analysis
We compared demographic and clinical characteristics of donor organs based on CoV+, CoV + COD, and CoVneg status as well as recipient characteristics based on receipt of these organs. We used Chi-square and Fisher's exact tests to compare the associations of categorical characteristics and student's t-tests and non-parametric Wilcoxon's twos-sided tests for continuous variables as appropriate. We generated a multivariable general linear model to compare the follow-up estimated Glomerular Filtration Rate (eGFR) between recipients of CoV+, CoV + COD, and CoVneg kidneys. We adjusted for covariates that were identified as potentially associated with follow-up eGFR a priori which included time posttransplantation, race/ethnicity, age at transplantation, body mass index, and either DCD or DBD donor. The statistical significance of variables in the model was evaluated based on Type-III p-values with a two-sided type-I error value of .05. Type-III tests evaluated the overall statistical significance of the applicable explanatory variable across individual levels of categorical variables. We also estimated the adjusted (least-squares) mean eGFR from these models for categorical variables. We generated Kaplan–Meier plots to test the association of donor CoV status with the development of COVID-19 diagnosis or SARS-CoV-2 detection posttransplantation. Kaplan–Meier plots were censored for graft failure and death and the last follow-up data was available. The association of time to recipient SARS-CoV positive status was tested with the log-rank test. All statistical analyses were conducted with SAS (v.9.4., Cary, NC).
3 RESULTS
From February 1, 2021 to January 31, 2022, there was 220 deceased donor kidney-only transplants with 115 transplants from 35 CoV+ and 33 CoV + COD donors and 105 transplants from 95 CoVneg donors.
3.1 SARS-CoV-2 positive donor characteristics
Characteristics of CoV+ donors with and without CoV + COD and comparative data of CoVneg donors are listed in Table 1. Those with CoV + COD were due to COVID-related lung injury, with six (19%) requiring venovenous extracorporeal membrane oxygenation (VV ECMO). Two CoV + COD donors tested negative for SARS-CoV-2 within 72 hours of organ donation and may have been considered “COVID resolved” by time from the first positive test but still were handled by the local OPO as a COVID-positive donor. Descriptive features of chest imaging (plain films and CT scans) were compatible with viral pneumonia in 100% of those with CoV + COD and 34% of CoV+ with other causes of death. The mean cycle threshold (Ct) for the 26 donors in whom it was reported was 29 (SD 8.6) with a lower mean Ct for CoV+ compared to CoV + COD, 28 versus 31.9, respectively. Cycle thresholds for eight (30.7%) donors were 35–41, for 11 donors (42.3%) were 25–34 and for seven donors (26.9%) were 10–24. The median time from the first positive SARS-CoV-2 test to organ procurement was 14 days (range 0–66) with longer median intervals for CoV + COD compared to CoV+ donors, 27 (range 10–66) versus 3 (range 0–44), respectively. Of the 64 kidneys with pre-transplant biopsies, only two had any significant findings, with one kidney having 14% glomerulosclerosis (GS), and one with 10%–30% tubular atrophy and moderate inflammation.
| Characteristic | CoV+ donors | CoV+ COD donors | CoVneg donors | p-value |
|---|---|---|---|---|
| N = 35 | N = 33 | N = 93 | ||
| Age, years, mean (SD) | 32 (12.1) | 40.4 (12.1) | 39.9 (13.2) | <.01 |
| Mean KDPI (SD) | 36.1 (22.1) | 40.6 (22.3) | 45.5 (24.7) | .12 |
| DCD (%) | 14 (40%) | 33 (100%) | 43 (46.2%) | <.01 |
| BMI kg/m2, mean (SD) | 27.9 (8.7) | 38.5 (10.4) | 30.5 (7.7) | <.01 |
| Race/ethnicity | .02 | |||
| White | 20 (57%) | 28 (85%) | 76 (82%) | |
| African American/Black | 9 (26%) | 1 (3%) | 13 (14%) | |
| Latino/Hispanic | 4 (11%) | 3 (9%) | 4 (4%) | |
| Other | 2 (6%) | 1 (3%) | 0 (0%) | |
| Cold ischemic time, h, mean (SD) | 22.9 (6.3) | 25.2 (6.6) | 22.2 (6.7) | .02 |
| Organ procurement locations | <.01 | |||
| Ohio | 7 (20%) | 2 (6%) | 56 (60%) | |
| Surrounding states (IN KY MI NY PA) | 15 (43%) | 6 (18%) | 29 (31%) | |
| Other statesa | 13 (37%) | 25 (76%) | 8 (9%) | |
| VV-ECMO | 0 | 6 (18%) | 1 (1%) | |
| Imaging | ||||
| Compatible w viral pneumonia | 12 (34%) | 33 (100%) | ||
| Normal | 9 (26%) | |||
| Abnormal/Other | 14 (40%) | |||
| SARS-CoV-2 RNA+, <72 h | 35 (100%) | 31 (94%) | ||
| Cycle threshold, mean, (SD), n = 18.8 | 28 (9.9) | 31.9 (5.1) | ||
| 10–24 | 6 (33.3%) | 1 (12.5%) | ||
| 25–34 | 6 (33.3%) | 5 (62.5%) | ||
| 35–41 | 6 (33.3%) | 2 (25%) | ||
| Time from first positive SARS-CoV-2 RNA to donation, days, median (range) | 3 (0–44) | 27 (10–66) |
- Abbreviations: BMI, body mass index; DCD, donation after cardiac death; KDPI, kidney donor index; VV, ECMO venovenous extra-corporeal membrane oxygenation.
- a Other states CoV+/CoV + COD/CoVneg donors: CA 3/0/1, CO 1/0/0, GA 0/1/1, IA 0/1/0, IL 0/0/1, KS 1/16/0, LA 0/0/1, MO 1/1/0, NC 1/1/0, NE 0/1/0, NJ 0/1/0, NV 1/0/0, OK 1/3/0, TX 2/0/0, WA 2/0/0, WI 0/0/1.
CoV + COD donors, compared to CoV+ and CoVneg donors, were more likely to be donation after cardiac death (DCD) (100% vs. 40% and 46.2%, p < .01) with longer cold ischemia times (CITs) (25.2 h vs. 22.9 h and 22.2 h, p = .02), and higher BMI (38.5 vs. 28.2 and 30.5 kg/m2, p < .01). CoV+ donors were younger (p < .01) and were less likely to be white (p = .02).
CoV+ and CoV + COD donors, compared to CoVneg donors, were significantly (p < .01) less likely to come from Ohio (17% and 9% vs. 60%) and more likely to come from more distant organ procurement sites (40% and 73% vs. 9%), including sites as far away as California and Washington State. Nineteen (27.9%), including two CoV+ and 17 CoV + COD donors were identified by two OPOs in the Kansas City area.
3.2 Recipient characteristics
Recipients of CoV+, CoV + COD, and CoVneg donors were of similar BMI, diabetes status and race/ethnicity (Table 2). However, recipients of CoV+ donor kidneys were younger and those with CoV + COD kidneys were older, p = .04. Both CoV+ and CoV + COD recipients were more likely to be pre-emptive (32.3%, 37.5%, and 16.2%, p = .01). For those that were not pre-emptive, recipients of CoV+ and CoV + COD donor kidneys experienced a shorter duration of dialysis (25.8 [IQR 16–43.6] months, 21.5 [IQR 12.4–31.1] months vs. 38.9 [22.9–60] months, p < .01). Recipients of CoVneg donors were somewhat less likely than those of CoV+ or CoV + COD donors to have been vaccinated or have documented resolved SARS-CoV-2 infection prior to transplantation (88% vs. 93.2% and 100%, p = .02). All the unvaccinated individuals received transplants prior to November 2021 when our center required vaccination as a condition of transplant eligibility.
| Characteristic | Recipients of CoV+ donors other COD | Recipients of CoV + COD donors | Recipients of CoVneg donors | p-value |
|---|---|---|---|---|
| N = 59 | N = 56 | N = 105 | ||
| Age, mean (SD) | 52.4 (14.3) | 58.4 (10.4) | 55.9 (12.8) | .04 |
| BMI kg/m2, mean (SD) | 29.2 (7.2) | 29.3 (5.4) | 29.6 (5.8) | .89 |
| Race/ethnicity | .23 | |||
| African American (%) | 12 (20.3%) | 15 (26.8%) | 37 (35.2%) | |
| White (%) | 37 (62.7%) | 34 (60.7%) | 59 (56.2%) | |
| Other (%) | 10 (17.0%) | 7 (12.5%) | 9 (8.6%) | |
| Diabetes (%) | 17 (28.8%) | 19 (33.9%) | 43 (40.9%) | .46 |
| Pre-emptive transplant (%) | 19 (32.2%) | 21 (37.5%) | 17 (16.2%) | .01 |
| Dialysis timea, months, median (IQR) | 25.8 [16–43.6] | 21.5 [12.4–31.1] | 38.9 [22.9–60.0] | <.01 |
| Wait list time, months, median (IQR) | 14.9 [3.9–22.1] | 8.0 [4.8–18.2] | 9.6 [3.0–29.8] | .63 |
| Prior vaccine and/or resolved COVID | 55 (93.2%) | 56 (100.0%) | 92 (88%) | .02 |
- a For non-preemptive transplant patients.
3.3 Transplant outcomes for recipients of CoV+ and CoVneg donors
Overall median follow-up was 5.7 months (IQR 2.7–8.3; range 1–12 months), but was shorter for recipients of CoV + COD than CoV+ and CoVneg donor kidneys (3.7 vs. 5.7 and 7.2 months, p < .001). There were no significant differences in most key posttransplant outcomes for recipients of CoV+ or CoV + COD compared to CoVneg donors (Table 3). This included patient death (0.9% and 0 vs. 3.7%; p = .29) and allograft loss (1.7% and 0 vs. 0; p = .35). Similarly, delayed graft function (10.3% and 21.8% vs. 21.9%; p = .16), allograft rejection (5.1%, 0 and 8.5%, p = .07), hospital length of stay (3 days, [IQR 2–4] and 3 days [IQR 3–4] vs. 3 days [IQR 2–4], p = .15), and 30 day hospital readmission rates (22.4% and 28.1% vs. 40.0%, p = .08) were no different.
| Characteristic | Recipients of CoV+ donors other COD | Recipients of CoV + COD donors | Recipients of CoVneg donors | p-valuea |
|---|---|---|---|---|
| N = 59 | N = 56 | N = 105 | ||
| Follow-up time, months, median [IQR] | 5.7 [3.1, 8.2] | 3.7 [1.6, 5.7] | 7.2 [3.7, 9.3] | <.001 |
| Delayed graft function (%) | 6 (10.3%) | 12 (21.8%) | 23 (21.9%) | .16 |
| Median LOS, days, [IQR] | 3 [2–4] | 3 [3–4] | 3 [2–4] | .15 |
| 30-day hospital readmission (%) | 13 (22.4%) | 19 (33.9%) | 42 (40.0%) | .08 |
| Allograft rejectionb | 3 (5.1%) | 0 | 9 (8.5%) | .07 |
| Allograft loss (not due to death) (%) | 1 (1.7%) | 0 (0%) | 0 (0%) | .35 |
| Death (%) | 1 (0.9%) | 0(0%) | 4 (3.7%) | .29 |
| Posttransplant COVID diagnosis (%) | 8 (13.6%) | 4 (7.1%) | 16 (15.2%) | .33 |
| Posttransplant COVID deaths | 0 | 0 | 2 | |
| sCr last follow-up, mg/dl (SD) | 1.66 (1.00) | 1.93 (0.60) | 1.67 (0.82) | .11 |
| eGFR at last follow-up (mL/min/m2)(SD) | 54.1(20.7) | 39.7 (15.3) | 51.4 (22.0) | .002 |
- a p-value from ANOVA or chi-square test for continuous or categorical variables, respectively; with exception of follow-up time, p-value based on nonparametric Wilcoxon test.
- b CoV + donors all with acute cellular rejection; for CoVneg donors for with ACR, 2 AMR, 3 mixed ACR, and AMR.
Graft function, as measured by eGFR at last follow-up, was lower for recipients of CoV + COD vs CoV+ and CoVneg donor kidneys (39.7 vs. 54.1 and 51.4 ml/min/m2, p = .002) though creatinine was not (1.66, 1.93 vs. 1.67 mg/dl, p = .11). On multivariable analysis (Table 4), recipient age at transplant, recipient BMI and DCD status were independently associated with worse eGFR, at last, follow-up. CoV + COD appeared associated with lower eGFR in this model but this did not reach statistical significance. Adjusted mean eGFR levels by donor CoV status and COD are displayed in Table 5 and demonstrate a significant impact on CoV + COD status on eGFR in the absence of controlling for other factors.
| Variable | Level | Parameter estimate | Type-III p-valuea |
|---|---|---|---|
| Donor COVID status | Negative | -reference level- | .13 |
| Positive not COD | 1.25 | ||
| Positive and COD | −6.14 | ||
| Time post-transplantation | (months) | 0.40 | .33 |
| Age at transplantation | (years) | −0.53 | <.001 |
| Race/ethnicity | White | -reference level- | .11 |
| Black | −3.95 | ||
| Other | −7.49 | ||
| Body mass index | kg/m2 | −0.49 | .02 |
| Donor type | DBD | -reference level- | .04 |
| DCD | −6.19 |
- a Type-III p-value tests the independent association of explanatory variables with eGFR.
| Variable | Level | Adjusted mean eGFR (ml/min/m2) | p-value |
|---|---|---|---|
| Donor CoV status | Negative | 49.3 | .008 |
| Positive not COD | 51.2 | ||
| Positive + COD | 40.6 | ||
| Race | White | 50.7 | .13 |
| Black | 47.1 | ||
| Other | 43.3 |
One recipient of a CoV+ donor kidney died 4 months posttransplant. He had pre-existing interstitial lung disease and experienced immediate peri-transplant worsening of lung dysfunction that initially improved but ultimately led to hypoxic respiratory failure. He tested negative for SARS-CoV-2 RNA from nasopharyngeal and bronchoalveolar lavage specimens, had no febrile illness and his pulmonary parenchymal changes were not characteristic of COVID-19. The kidney was from a 40-year-old brain-dead donor with newly detected SARS-CoV-2 detection within 72 h of donation, a clear chest X-ray, and no reported COVID-like symptoms. The recipient of the mate kidney experienced no post-operative complications and the allograft continues to function well 11 months posttransplant.
3.4 SARS-CoV-2-related posttransplant outcomes
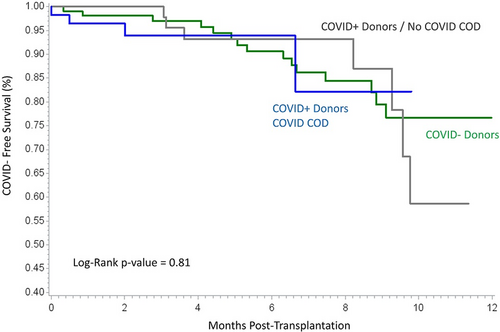
Posttransplant SARS-CoV-2 diagnoses (13.6%, 7.1% vs 15.2%, p = .89) and time from transplant to SARS-CoV-2 diagnoses (Figure 1) were no different between recipients of CoV+, CoV + COD, and CoVneg donors, respectively. There were two deaths from COVID-related hypoxic respiratory failure in the CoV-negative donor group occurring 4 and 5 months from transplant. The earliest posttransplant SARS-CoV-2 diagnosis in a recipient of CoV+ donor kidney was 15 days, detected on routine readmission testing. He was admitted due to loss of social support and he had no respiratory symptoms, a clear chest X-ray, and likely community exposure.
4 DISCUSSION
These comparative data demonstrate very good outcomes for over 100 kidney transplant recipients from CoV+ and CoV + COD donors, recipients that may not have otherwise received transplant offers over the year of study. It also demonstrates the safety of organ transplantation from donors at diverse stages of SARS-CoV-2 infection, including those with active infection and with COVID-19 as the cause of death. This novel information is clinically valuable as it provides another opportunity to minimize organ discard and expand the already contracted deceased donor pool.
Our early assessment of the low risk for SARS-CoV-2 transmission from extra-pulmonary organs to transplant recipients informed a more aggressive approach to offering such organs to kidney transplant candidates. As early as May of 2020, Kates et al. made a very strong case for use of extra-pulmonary organs from SARS-CoV-2 infected donors.27 Their arguments over time continued to align with empirical evidence. By early 2021, it was reassuring that after millions of worldwide infections, evidence for productive infection of extra-pulmonary organs was only circumstantial with no consistent reports of intact virions, direct viral damage, or replication-competent virus at these sites.3-5, 28 While changes in viral pathogenesis could emerge with SARS-CoV-2 evolution, there has been no signal for alternative mechanisms of SARS-CoV-2 transmission for any variants during our study period and an overall reduction in disease severity.29
We saw no negative impact on patient survival and no clinically observable evidence for virus transmission from the allograft to our recipients. If both kidneys from 45 CoV+ or CoV + COD donors came to our center, one recipient could act as a control for adverse outcomes should they occur. One death occurred in a recipient of a CoV+ donor organ during the study period (compared to four deaths of recipients of CoVneg donor kidneys). The recipient of the mate kidney experienced no post-operative complications and the allograft continues to function well 11 months posttransplant.
SARS-CoV-2 infection rates were no different between recipients of CoV+ and CoVneg donor kidneys. Nearly all cases of COVID-19 that occurred posttransplant occurred during evident periods of viral transmission in the community. The SARS-CoV-2 cases that emerged earlier posttransplant were scrutinized to assess for possible donor-derived transmission, though our clinical tools to do so were limited. While donor-derived infection could not entirely be ruled out, these cases were asymptomatic, detected in the upper airway, demonstrated no lower airway disease on imaging, and were timed with likely community acquisition.
As observed previously, many of the earliest CoV+ donors in our series and reported elsewhere had very little evidence for active infection and may simply have had residual RNA shedding following resolved infection. Throughout the year we accepted CoV+ donors with active COVID-19, as evidenced by low cycle thresholds and characteristic pulmonary infiltrates. We also accepted a large number of kidneys from donors that had died of COVID-related respiratory failure. Given the time from the first positive SARS-CoV2 RNA detection of up to 66 days, some of these donors likely had resolved SARS-CoV-2 infection making virus transmission from these kidneys even less likely. Thus, the meaning of SARS-CoV-2 detection in this study may be quite broad and difficult to clearly categorize. This may actually be helpful in that OPOs and transplant centers needn't be excessively proscriptive in considering these donors.
Since we considered SARS-CoV-2 transmission from kidney transplantation to be unlikely, we did not offer monoclonal antibodies, remdesivir or convalescent plasma specifically intended to mitigate this theoretical risk. Such use would have been off-label or outside of the emergency use authorizations since no infection was documented in the recipients. We encouraged vaccination early and later required vaccination for all our transplant candidates, though this was intended to reduce the risk of disease acquired in the community. Our induction and maintenance immunosuppression regimens were not altered or modified for recipients of CoV+ or CoV + COD donors.
We assessed allograft quality similarly for CoV+, CoV + COD, and CoVneg donors. Whether or not there should be additional scrutiny for assessing organs from CoV+ and CoV + COD donors is unclear. Kidney injury is a common occurrence in those hospitalized for COVID-related illness and is associated with greater disease severity.6, 30, 31 In autopsy series of those dying from COVID-19 and live kidney biopsies in those with AKI, thrombotic microangiopathy, collapsing glomerulopathy and diffuse tubular injury are most commonly described.32 However, the comorbidities associated with severe COVID-19 also impact these pathologies. Adjusting for such comorbidities, 30-day survivors of milder forms of COVID-19 may still be at increased risk for adverse kidney outcomes.7 Even in the absence of detectable kidney dysfunction, there is the possibility of early cytokine-mediated vascular or inflammatory injury that may manifest later. However, such events have not been elucidated either pathologically or clinically and remain theoretical.
Overall, allograft outcomes in our series were not significantly different between CoV+ and CoVneg donors. CoV+ donors were younger, less likely to be white, and more likely to come from outside Ohio but otherwise had similar characteristics to CoVneg donors, including similar KDPI. That functional renal outcomes are influenced by the severity of COVID-19 disease raises particular interest in allograft outcomes from CoV + COD donors. Our analysis did find a difference in eGFR at the last follow-up for recipients of kidneys from donors dying from COVID-19. Though this was not significant in multivariable analysis, it is important and requires further consideration. The shorter follow-up time for this group could indicate that eGFR will worsen further or has not yet optimally peaked posttransplant. Thus, additional follow-up is crucial. That CoV+ COD donors were all DCD, had longer CITs, higher BMIs, and more prolonged illness prior to donation likely influenced graft function, despite attempts to control for these in our multivariable analysis. Interestingly, the DCD status did not lead to a statistically meaningful difference in delayed graft function typically seen from DCD donors.33, 34 This is likely due to other confounding, but favorable, factors in the CoV+ donor group. DCD was, however, highly associated with lower eGFR at the last follow-up, independent of CoV donor status, and as part of KDPI remains an important factor in allograft selection.
The finding of longer cold ischemia times for the CoV + COD donor kidneys is notable and is informed by the unique aspects of organ procurement from donors during these years of the pandemic. Most of the CoV+ donors were identified outside of our immediate region and from much farther distances compared to CoVneg donors. OPOs in some regions appeared to be willing to evaluate CoV+ donors, particularly during COVID-19 surges. However, the local transplant centers that are served by these OPOs may not have been pursuing these donors. It is likely that such a widespread mismatch between OPO and transplant center interest in CoV+ organs will diminish over time and reduce cold ischemia times for such organs. Given historical data, the impact of longer CITs combined with DCD on allograft function in CoV + COD donors will continue to be assessed.35
Over half of our center's kidney-only transplants came from CoV+ or CoV + COD donors during the period of study. Very few transplant candidates declined the opportunity for transplantation from any CoV+ donor. To justify the then-unknown calculated risk, we offered CoV+ and CoV + COD kidneys to recipients who were more recently activated on the transplant list and who would have otherwise waited a long time to receive an organ offer. Thus, recipients of CoV+ and CoV + COD kidneys were more likely to be pre-emptive. Similarly, for those on dialysis, the duration of dialysis was shorter than for recipients of CoVneg donors. While these are favorable factors in regards to allograft outcome we found no significant effect of these variables on the impact of donor CoV status on eGFR when added to our multivariable model (data not presented).
This study has several limitations. First, it compares two SARS-CoV-2 positive groups with distinct terminal pathophysiology to CoVneg donors. Terminal COVID-19 with associated respiratory failure as a cause of death is more likely to affect allograft function than to transmit active SARS-CoV-2 given the potential and proven time from infection onset to organ donation. While many could now be considered “resolved infection,” these positive tests continued to raise concerns among OPOs and transplant programs. Another important limitation is the limited period of follow-up to assess allograft-related outcomes, particularly for recipients of CoV + COD donors transplanted during the early Omicron surge. A longer follow-up is required to arrive at definitive conclusions. Additionally, our numbers did not allow for a propensity-matched CoVneg donor control group and our multivariable model could not account for many baseline differences in donor and recipient characteristics that may differentially influence patient and allograft outcomes.
Overall, we found that kidney transplantation from CoV+ donors with otherwise healthy appearing kidneys was safe and successful with up to 1 year of follow-up compared to those from CoVneg donors. This appears to be true for kidneys from donors at different stages of SARS-CoV-2 infection, recognizing that follow-up from those transplants performed from CoV+ donors with COVID-related causes of death during the Omicron surge is limited. This may also inform the transplantation of hearts and livers from such donors, settings where transplantation is more urgently lifesaving. We advocate for more widespread consideration of SAR-CoV-2 positive donors and donors dying of COVID-19 for kidney transplantation using typical tools required to assess organ quality and very close follow-up of recipient and allograft outcomes.
ACKNOWLEDGMENTS
The authors would like to thank those involved in data management and study administration.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




