Humanized anti-IL-26 monoclonal antibody as a novel targeted therapy for chronic graft-versus-host disease
Abstract
IL-26 is a Th17 cytokine, with its gene being absent in rodents. To characterize the in vivo immunological effects of IL-26 in chronic systemic inflammation, we used human IL26 transgenic (hIL-26Tg) mice and human umbilical cord blood mononuclear cells (hCBMC) in mouse allogeneic-graft-versus-host disease (GVHD) and chronic xenogeneic-GVHD model, respectively. Transfer of bone marrow and spleen T cells from hIL-26Tg mice into B10.BR mice resulted in GVHD progression, with clinical signs of tissue damage in multiple organs. IL-26 markedly increased neutrophil levels both in the GVHD-target tissues and peripheral blood. Expression levels of Th17 cytokines in hIL-26Tg mice-derived donor CD4 T cells were significantly increased, whereas IL-26 did not affect cytotoxic function of donor CD8 T cells. In addition, granulocyte-colony stimulating factor, IL-1β, and IL-6 levels were particularly enhanced in hIL-26Tg mice. We also developed a humanized neutralizing anti-IL-26 monoclonal antibody (mAb) for therapeutic use, and its administration after onset of chronic xenogeneic-GVHD mitigated weight loss and prolonged survival, with preservation of graft-versus-leukemia effect. Taken together, our data elucidate the in vivo immunological effects of IL-26 in chronic GVHD models and suggest that a humanized anti-IL-26 mAb may be a potential therapeutic agent for the treatment of chronic GVHD.
Abbreviations
-
- A20-luc
-
- firefly luciferase-transfected A20
-
- allo-GVHD
-
- allogeneic-GVHD
-
- allo-HSCT
-
- allogeneic hematopoietic stem cell transplantation
-
- BAC
-
- bacterial artificial chromosome
-
- BM
-
- bone marrow
-
- CNS
-
- conserved noncoding sequence
-
- G-CSF
-
- granulocyte-colony stimulating factor
-
- GVHD
-
- graft-versus-host disease
-
- GVL
-
- graft-versus-leukemia
-
- hCBMC
-
- human umbilical cord blood mononuclear cells
-
- hIL-26Tg
-
- human IL26 transgenic
-
- mAb
-
- monoclonal antibody
-
- NETs
-
- neutrophil extracellular traps
-
- NOG
-
- NOD/Shi-scidIL2rγnull
-
- TCD-BM
-
- T-cell-depleted bone marrow
-
- xeno-GVHD
-
- xenogeneic GVHD
1 INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a well-established procedure for hematological malignancies or bone marrow (BM) failure syndromes,1-3 with acute and chronic graft-versus-host disease (GVHD) due to an immunological attack on target recipient organs by donor allogeneic T cells being significant complications. While suppression of donor allogeneic T cells is highly effective for preventing GVHD, complications such as loss of graft-versus-leukemia (GVL) effect or increased opportunistic infections can occur.4 In-depth understanding of the molecular mechanisms involved in human GVHD will improve allo-HSCT clinical outcome.
IL-26 belonging to the IL-10 cytokine family,5, 6 is conserved in several vertebrate species but not found in mice and rats.7 IL-26 is known as a Th17 cytokine, while NK cells, macrophages, bronchial epithelial cells, and synoviocytes have capacity to produce IL-26.8-11 IL-20RA/IL-10RB heterodimer is an IL-26 receptor, and IL-26 binding to IL-20RA/IL-10RB results in functional activation via STAT3 phosphorylation.12 However, IL-26 can activate human monocytes and NK cells,8, 13 despite the lack of IL-20RA expression on these cell types.14 IL-26 also acts on vascular endothelial cells to stimulate angiogenesis and activates EGFR-tyrosine kinase inhibitor-associated bypass pathway to promote drug resistance in triple-negative breast cancer cells despite a lack of IL-20RA expression.11, 15 These findings strongly suggest the existence of an unidentified IL-26 receptor besides IL-20RA/IL-10RB. Furthermore, IL-26 is a cationic and amphipathic cytokine with characteristics of an antimicrobial peptide,16, 17 capable of binding to DNA/RNA released from bacteria or dying cells or neutrophil extracellular traps (NETs), triggering inflammatory cytokine production from plasmacytoid dendritic cells, monocytes, and neutrophils in a TLR9 or STING- and inflammasome-dependent manner,16, 18 indicating the diverse mechanism of action of IL-26.
Without accessible animal models due partly to the absence of the IL26 gene in mice, IL-26 role in inflammatory disorders is not clearly characterized. Utilizing human IL26 bacterial artificial chromosome (BAC) transgenic (hIL-26Tg) mice,19, 20 we recently investigated the in vivo effects of IL-26 in acute local inflammation models and showed that vascularization and immune cell infiltration were enhanced in the skin of imiquimod-applied hIL-26Tg mice.15, 21 These findings strongly suggest that IL-26 may represent a novel therapeutic target for inflammatory disorders, although IL-26 role in chronic inflammation still needs clarification. In the murine models utilizing hIL-26Tg mice, human IL-26 is not expressed constitutively but is induced only when the cells capable of expressing IL-26 are activated, which resembles the human condition. However, a potential drawback of the hIL-26 BAC Tg mouse model is that the expression level of human IL-26 in murine CD4 T cells is quite low as compared with the activated human CD4 T cells,20 resulting in suboptimal biological effects of human IL-26 in the inflammation models involving hIL-26Tg mice. For these reasons, inflammation models utilizing human T cells are essential to investigate the inherent functions of human IL-26.
Due to the reasons above, in the present study, we examined mouse allogeneic (allo)-GVHD model utilizing hIL-26Tg mice and chronic xenogeneic (xeno)-GVHD model utilizing human umbilical cord blood mononuclear cells (hCBMC) to evaluate the in vivo immunological effects of IL-26 in the pathology of chronic systemic inflammation, and created a humanized neutralizing anti-IL-26 monoclonal antibody (mAb) based on our recently developed murine mAb22 as a potential treatment for chronic GVHD.
2 METHODS
2.1 Human samples
Serum samples were obtained from nine mild acute skin GVHD patients (6 males and 3 females, 2 grade I and 7 grade II, age 47.56 ± 9.62) and six moderate/severe chronic lung GVHD patients (4 males and 2 females, 2 severe, and 4 moderate, age 48.33 ± 15.04). Serum was collected from patients following GVHD diagnosis at Sapporo Medical University. Serum was collected from 12 healthy volunteers (8 males and 4 females, age 46.83 ± 11.47) at Juntendo University. All serum samples were stored at −80°C. Mononuclear cells isolated from human cord blood with Ficoll density gradation method were purchased from RIKEN BioResource Center.
2.2 Mice
Female B10.BR (H-2k) and NOD/Shi-scidIL2rγnull (NOG) (H-2d) mice were purchased from Sankyo Labo-Service and In-Vivo Science, respectively. C57BL/6 (H-2b) mice carrying a 190-kb BAC transgene with human IFNG and IL26 gene (hIL-26Tg) and a BAC Tg-deleting conserved noncoding sequence (CNS) positioned at 77 kb upstream of the IFNG transcription start site (ΔCNS-77Tg) were developed in Dr. Aune's laboratory.19, 20 Human IL-26 expression was completely abrogated in ΔCNS-77Tg mice.20 Although both hIL-26Tg mice and ΔCNS-77Tg mice-derived CD4 T cells were capable of producing human IFN-γ, it has been reported that human IFN-γ does not transduce intracellular signals through murine IFNGR nor exhibit biological activity.23 All mice used in this study were housed in a specific pathogen-free facility in micro-isolator cages, and used at 10–12 weeks of age.
All other information is detailed in Supplementary methods.
3 RESULTS
3.1 Exacerbation of systemic manifestations of allo-GVHD by IL-26
We first used hIL-26Tg mice for a complete MHC-mismatched allo-GVHD model, having previously demonstrated that human IL-26 can act on both murine and human cells.11, 15, 24 ΔCNS-77Tg mice lacking human IL-26 transcription were used as controls. The basic characteristics of spleen T cells in hIL-26Tg mice were generally similar as those in control mice (Figure S1). Transplantation of T cell-depleted bone marrow (TCD-BM) alone hardly affected survival and weight of B10.BR recipients (Figure 1A,B). In contrast, acute weight loss occurred until one-week post-transplant of donor BM plus splenic T cells, followed by a slight body weight recovery and then a gradual decrease due to allo-GVHD. B10.BR mice receiving hIL-26Tg mice-derived BM and splenic T cells (hIL-26Tg-B10.BR mice) exhibited significantly decreased overall survival (p = .0215) and body weight, compared to recipients transplanted with ΔCNS-77Tg mice-derived BM and splenic T cells (ΔCNS-77Tg-B10.BR mice) (Figure 1A,B). Blood leukocytes of recipient mice were mostly from donor-derived H-2Db+H-2Dk− cells (Figure 1C). Although there was no difference in donor CD4 and CD8 T cell expansion between allo-GVHD groups, donor granulocyte levels were apparently increased in hIL-26Tg-B10.BR mice compared to ΔCNS-77Tg-B10.BR mice (Figures 1C, Figure S2).
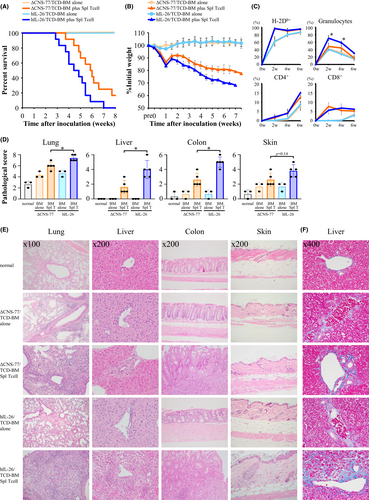
GVHD manifestations were hardly observed in B10.BR mice receiving TCD-BM alone (Figure 1D,E). In contrast, histiocytes in pulmonary alveoli, portal inflammatory cell infiltration around portal vein, and severe colitis with ulceration were observed in hIL-26Tg-B10.BR mice, with significantly higher pathological scores of these organs compared to those of control mice, whereas skin GVHD manifestations were moderate in this model (Figures 1D,E, Figure S3). Moreover, collagen deposition was clearly observed in the portal area of the liver of hIL-26Tg-B10.BR mice (Figure 1F). Considering the survival period of recipient mice after transplantation, the manifestation of colon and liver GVHD and lung inflammation, and the fibroproliferation in the liver, this allo-GVHD model appears to display the characteristics of both acute and chronic GVHD. Taken together, our data indicate that IL-26 further exacerbates systemic GVHD progression and severity.
3.2 IL-26-mediated enhancement of neutrophil levels and augmentation of Th17 response
Our next studies examined the mechanisms involved in IL-26-mediated allo-GVHD. CD45+ leukocytes were localized in the lung of normal B10.BR or B10.BR recipients receiving TCD-BM alone while being at low levels in the liver. Both allo-GVHD groups demonstrated increased levels of donor leukocytes in the lung and particularly in the liver (Figure 2A). There was a higher percentage of CD4 T cells than CD8 T cells in donor leukocytes in B10.BR mice receiving TCD-BM alone, with the ratio reversed in the allo-GVHD mice (Figure 2B). The percentage of donor CD4 and CD8 T cells in hIL-26Tg-B10.BR and ΔCNS-77Tg-B10.BR mice were similar, whereas neutrophil levels were markedly increased in lung, liver, spleen, and colon of hIL-26Tg-B10.BR mice, as well as a trend toward an enhanced level of donor monocytes and macrophages (Figure 2B, Figures S4 and S5). Meanwhile, B cell percentage was prominently decreased in the allo-GVHD mice as compared with recipients receiving TCD-BM alone (Figure 2B, Figure S6). We next evaluated the effect of anti-mouse Ly6G mAb to determine whether the increased neutrophil levels in the GVHD-target organs from hIL-26Tg-B10.BR mice were associated with GVHD exacerbation. Although anti-Ly6G mAb administration did not result in complete neutrophil depletion, levels of neutrophil infiltration in the lung and liver of hIL-26Tg-B10.BR mice were decreased, concurrent with a trend toward reduced allo-GVHD-associated lethality (p = .1546) and weight loss (Figure S7). These results suggest that the increased neutrophil levels in the GVHD-target organs are at least partially responsible for GVHD exacerbation.
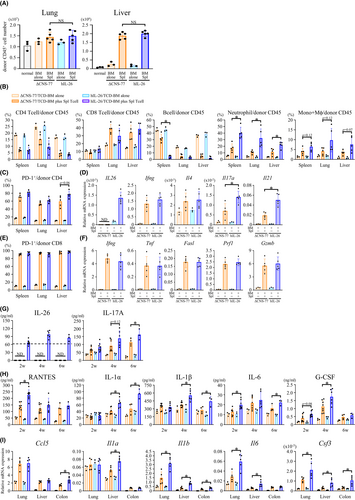
In contrast with mice receiving TCD-BM alone, most of the donor CD4 and CD8 T cells in the allo-GVHD mice expressed PD-1, suggesting a TCR-stimulated phenotype (Figure 2C,E). Human IL26 mRNA expression was observed only in donor CD4 T cells transplanted from hIL-26Tg but not ΔCNS-77Tg mice (Figure 2D). Expression levels of all the genes examined in donor CD4 and CD8 T cells purified from allo-GVHD mice were higher than from mice receiving TCD-BM alone. Mouse Il17a and Il21 levels in donor CD4 T cells in the liver of hIL-26Tg-B10.BR mice were significantly higher than those from ΔCNS-77Tg-B10.BR mice, with similar levels of effector molecules in donor CD8 T cells from hIL-26Tg-B10.BR mice and control recipients (Figure 2D,F). Higher expression level of mouse IL-17A in donor CD4 T cells in the liver of hIL-26Tg-B10.BR mice was also confirmed by flow cytometry (Figure S8). Similar results were observed for lung and spleen donor T cells (data not shown). Plasma levels of human IL-26 and mouse IL-17A were similar to those shown in Figure 2D,G.
Inflammatory factors highly associated with human IL-26 expression were identified by multiplex assays. Among 23 cytokines and chemokines evaluated, plasma levels of mouse RANTES, IL-1α, IL-1β, IL-6, and granulocyte-colony stimulating factor (G-CSF) in hIL-26Tg-B10.BR mice were significantly higher than those in ΔCNS-77Tg-B10.BR mice (Figure 2H). Furthermore, quantitative RT-PCR analyses confirmed the enhanced mRNA expression of these genes, particularly Il1b, Il6, and Csf3, in the GVHD-target organs of hIL-26Tg-B10.BR mice (Figure 2I). Our data hence indicate that IL-26 markedly increases neutrophil levels in GVHD-target tissues and peripheral blood, and augments Th17 response associated with enhanced levels of G-CSF, IL-1β, and IL-6.
3.3 IL-26-mediated augmentation of Th17 response and G-CSF production
Since IL-26 and DNA have synergistic effect on IL-1β and IL-6 production,18 we next analyzed Il1b and Il6 expression in mouse myeloid cells. Human IL-26 plus mouse genomic DNA stimulated both Il1b and Il6 mRNA expression and IL-1β and IL-6 production from primary mouse splenic CD11b+ cells (Figure 3A,B), but not Il12b and Il23a expression (data not shown). Similar results were observed for the mouse macrophage cell line RAW264.7 (Figure S9).
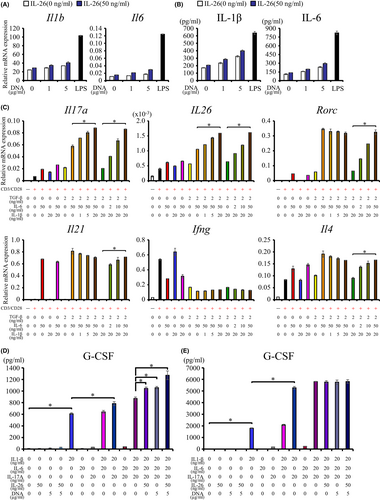
Other cytokines besides TGF-β and IL-6 are involved in the functional maturation and maintenance of Th17 cells.25-28 Since we demonstrated that IL-26 exposure was associated with enhanced IL-1β and IL-6 expression, we next examined IL-1β and IL-6 effect on Th17 polarization and activation. Exogenous mouse IL-1β and IL-6 augmented mouse Il17a and human IL26 expression, respectively, in hIL-26Tg mice-derived splenic CD4+ T cells costimulated via CD3 and CD28 in the presence of mouse TGF-β1, IL-1β, and IL-6 (Figure 3C). In contrast, stimulation with TGF-β1 and IL-6 but not IL-1β was essential to induce CD4+ T cell Rorc expression, the master transcription factor driving Th17 cell differentiation (Figure 3C), while Il21 expression was regulated by IL-6 alone (Figure 3C). TGF-β1 and IL-6 stimulation suppressed Ifng expression while slightly enhanced Il4 expression (Figure 3C). Mouse cytokine and Rorc levels were similar in ΔCNS-77Tg mice-derived and hIL-26Tg mice-derived splenic CD4+ T cells, while human IL26 expression was never observed in ΔCNS-77Tg mice-derived CD4+ T cells in all stimulatory conditions (data not shown). These results indicate that both IL-1β and IL-6 are essential for the marked increase in mouse IL-17A and human IL-26 expression in CD4+ T cells of hIL-26Tg mice.
We also examined the association between IL-26-mediated positive-feedback and G-CSF expression, a critical factor for granulopoiesis.29 Stimulation with mouse IL-1β dramatically induced G-CSF production from RAW264.7 cells, with additional enhancement by mouse IL-17A, genomic DNA, and human IL-26 (Figure 3D). Meanwhile, mouse IL-1β and IL-17A synergistically enhanced G-CSF production from NIH3T3 cells, with minimal effect by mouse IL-6, genomic DNA, and human IL-26 (Figure 3E).
3.4 Development of novel humanized anti-IL-26 mAb
To develop a novel IL-26-targeted therapy, we have succeeded in developing humanized mAb h69-10 with strong binding affinity and neutralizing activity against IL-26 as compared with the original murine mAb m69-10 (Figure S10).
3.5 Suppression of xeno-GVHD progression by anti-IL-26 mAb treatment
Acute GVHD is caused by T cells within the original stem cell transplant, whereas chronic GVHD is theoretically caused by allo- (auto-) reactive T cells that have matured through the host thymus. We previously established a chronic xeno-GVHD model,24 which has two inherent advantages that allow us to investigate the therapeutic effect of anti-IL-26 mAb. One is human IL-26 expression level in human CD4 T cells is much higher than hIL-26Tg mice-derived CD4 T cells, and the other is disease progression is moderate as compared with the hIL-26Tg-B10.BR allo-GVHD model. m69-10 or h69-10 treatment following the appearance of GVHD clinical symptoms on day +28 markedly increased overall survival of hCBMC-NOG mice (Figure 4A), with stable body weight for up to 7 weeks post-transplantation (Figure 4B). In this model, recipient-derived hematopoietic stem cells were not destroyed completely by sublethal irradiation, resulting in the development of chimeric mice. Human leukocytes were mainly composed of T cells, whereas mouse leukocytes were mostly granulocytes and monocytes/macrophages. Although anti-IL-26 mAb administration did not affect donor CD4 and CD8 T cell expansion, there was a trend toward a decrease in the percentage of mouse granulocytes in hCBMC-NOG mice receiving m69-10 or h69-10 (Figure 4C).
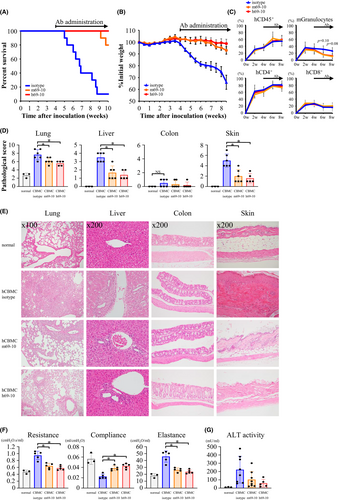
Although consolidation in lung, regenerative change in bile duct epithelium, cholestasis in hepatocytes, and acanthosis, follicular dropout, sclerosis of reticular dermis, and fat loss in skin were often observed in hCBMC-NOG mice receiving control IgG, progression of these systemic GVHD symptoms were prominently suppressed in m69-10 or h69-10-administered hCBMC-NOG mice, with hardly any GVHD manifestation observed in colon tissues of all mice (Figure 4D,E, Figure S11). Furthermore, significant decrease in resistance and elastance, and increase in compliance were observed in pulmonary functions of hCBMC-NOG mice receiving anti-IL-26 mAb (Figure 4F). Serum alanine transaminase activity was also elevated in mice treated with control IgG compared to anti-IL-26 mAb (Figure 4G). These data indicate that both h69-10 and m69-10 treatment significantly impeded chronic GVHD development.
3.6 Anti-IL-26 mAb-mediated suppression of T cell and neutrophil infiltration, Th17 response, and fibroproliferation
Administration of h69-10 or m69-10 markedly reduced the total number of donor leukocytes in both lung and liver of hCBMC-NOG mice, while the percentage of CD4 and CD8 T cells in donor leukocytes of mice receiving anti-IL-26 mAb was nearly identical to that of mice receiving control IgG, indicating that anti-IL-26 mAb treatment decreased the absolute number of donor CD4 and CD8 T cells in the GVHD-target organs (Figure 5A–C). Anti-IL-26 mAb administration also significantly reduced the total number of recipient leukocytes in lung and the percentage of neutrophils in recipient leukocytes in the spleen, lung, or liver of hCBMC-NOG mice, indicating that anti-IL-26 mAb treatment reduced neutrophils systemically, but particularly in lung of hCBMC-NOG mice (Figure 5A–C, Figure S12).
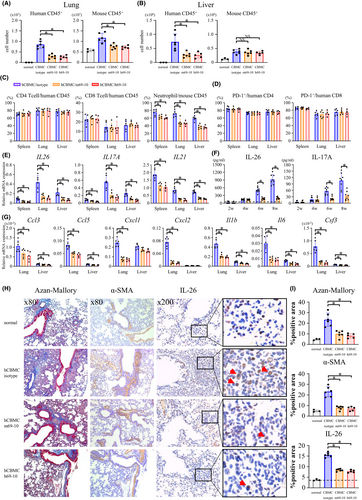
Most of donor CD4 and CD8 T cells in hCBMC-NOG recipients expressed PD-1, suggesting a TCR-stimulated phenotype (Figure 5D). In addition, anti-IL-26 mAb treatment markedly decreased human IL26, IL17A, and IL21 but not IFNG and IL4 expression levels in human CD4 T cells (Figure 5E, Figure S13), plasma levels of human IL-26 and IL-17A (Figure 5F), and mRNA expression levels of mouse Ccl3, Ccl5, Cxcl1, Cxcl2, Il1b, Il6, and Csf3 in lung or liver of hCBMC-NOG recipients (Figure 5G).
While the lung of hCBMC-NOG mice receiving control IgG manifested significant peribronchiolar and perivascular collagen deposition and enhanced α-smooth muscle actin-positive myofibroblast level as compared with normal NOG mice (Figure, 5H,I, Figure S14), levels of collagen deposition, myofibroblasts as well as IL-26+ cells were prominently decreased in the lung of anti-IL-26 mAb-treated hCBMC-NOG mice (Figure 5H,I). Taken together, our data indicate that anti-IL-26 mAb treatment significantly suppresses T cell and neutrophil infiltration into GVHD-target organs, Th17 response, and fibroproliferation.
3.7 Maintenance of GVL effect in hCBMC-NOG mice receiving anti-IL-26 mAb treatment
Since GVHD and GVL are highly linked immune reactions,30 our next studies evaluated the potential influence of anti-IL-26 mAb on GVL effect. To stably develop tumor-disseminated recipient mice, firefly luciferase-transfected A20 (A20-luc) cells were inoculated on day +28 after hCBMC transplantation, followed by anti-IL-26 mAb treatment (Figure 6A). NOG mice inoculated with A20-luc alone displayed prominent tumor proliferation (Figure 6B,C), succumbing to high tumor burden within 3 weeks post-tumor inoculation. hCBMC-NOG recipients receiving control IgG exhibited clinical symptoms of GVHD such as weight loss and alopecia, although tumor progression was clearly suppressed, possibly by human T cells. In contrast, anti-IL-26 mAb treatment suppressed both tumor progression and GVHD manifestations (Figure 6B,C).
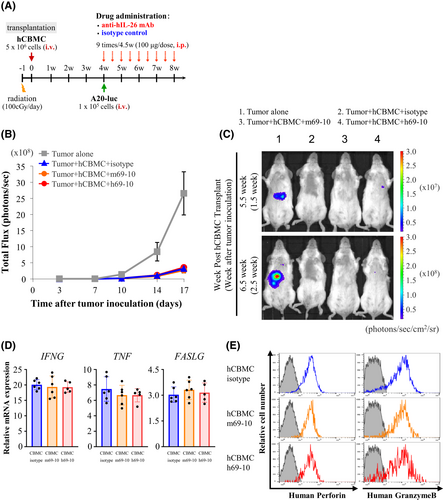
Meanwhile, mRNA expression levels of IFNG, TNF, FASLG, and protein expression levels of perforin and granzyme B as effector molecules in human CD8 T cells isolated from the spleen of hCBMC-NOG recipients following 3 weeks of anti-IL-26 mAb were comparable to mice treated with control IgG (Figure 6D,E). Taken together, our data indicate that effective GVL function is preserved in IL-26 mAb-treated recipients, possibly due to the maintenance of donor CD8 T cell cytotoxic functions.
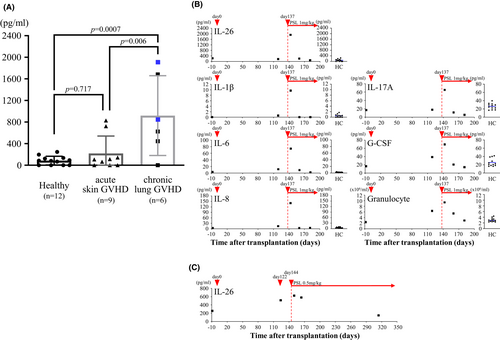
3.8 Elevated serum IL-26 level in GVHD patients
Extending our findings to the clinical setting, we found that two of nine patients with mild acute skin GVHD (829.1 and 723.1 pg/mL) and three of six chronic lung GVHD patients (1913.8, 1683.4, and 851.9 pg/mL) exhibited markedly high IL-26 levels, and the mean value of chronic lung GVHD patients (919.7 ± 739.5 pg/mL, n = 6) was significantly higher as compared with acute skin GVHD patients (219.9 ± 320.9 pg/mL, n = 9) and healthy controls (85.6 ± 80.8 pg/mL, n = 12; Figure 7A). Time-course analysis was conducted for one patient with severe chronic lung GVHD manifested by pneumothorax and pneumomediastinum at 137 days post-transplantation. Along with increased levels of neutrophils and several inflammatory cytokines, IL-26 serum level was markedly increased by day +143 with the onset of chronic GVHD, with a significant decrease by day +160 following 3 weeks of prednisolone treatment (from 1913.8 to 198.6 pg/mL), associated with improved clinical symptoms (Figure 7B). In contrast, serum IL-26 level in another patient with moderate chronic lung GVHD was hardly affected by 3 weeks of prednisolone treatment (from 624.1 to 578.3 pg/mL; Figure 7C). Our data indicate that serum IL-26 level is elevated at least in some chronic GVHD patients.
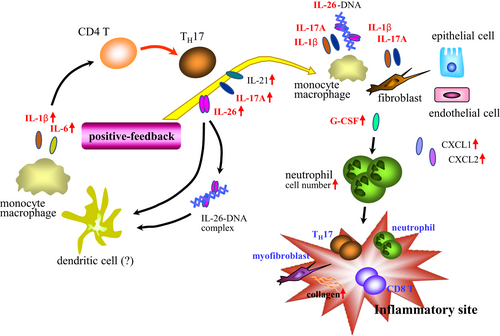
Based on our findings, Figure 8 depicts a schematic of the immunological effects of IL-26 in the pathophysiology of chronic systemic inflammation.
4 DISCUSSION
Although IL-26 is known as a Th17 cytokine, its precise role in inflammatory disorders is not fully understood due to the deficiency of IL26 in mice. Our group has previously shown that CD26 is a key costimulatory molecule for inducing IL-26 production from human CD4 T cells, and IL-26 is strongly associated with lung fibrosis in a chronic xeno-GVHD model.24 In this present paper, we evaluate the in vivo immunological effects of IL-26 on the level and composition of immune cells, T cell phenotypes, and inflammatory cytokine/chemokine expression utilizing hIL-26Tg mice and hCBMC. Furthermore, we demonstrate that humanized neutralizing anti-IL-26 mAb significantly impedes the development of chronic xeno-GVHD with preservation of GVL effect.
The xeno-GVHD model has several artificial characteristics. For example, almost all the human leukocytes engrafted in the recipient NOG mice are T cells and B cells, while neither human NK cells, antigen-presenting cells nor granulocytes have normal maturation and differentiation in this model. In addition, as human IFN-γ has been reported not to transduce signals through murine IFN-γ receptor,23 it is possible that several human effector molecules may not regulate murine cell functions as compared with human cells. In human, IL-26 can be produced not only from CD4 T cells but also from other cell types,8-11 while the cellular mechanisms involved in regulating IL-26 transcription have not been fully elucidated, including detailed characterization of IL-26-associated transcription factors or promoter regions. For these reasons, we utilized BAC Tg mice carrying a 190-kb that includes sequences upstream and downstream of the human IL-26 transcription region. In this murine model, human IL-26 is induced only when the cells capable of expressing IL-26 are activated, although the expression level of human IL-26 in murine cells is quite low as compared with activated human CD4 T cells. For these reasons, inflammation models utilizing human T cells are essential to investigate the inherent functions of human IL-26. Cross-species models have imperfections that need to be addressed, but we also believe that they can be used to improve our knowledge of human immunology, particularly for detailed analysis of genes that are deficient in mice or have different functions between human and mice. Of note, in the present study, we investigated the in vivo immunological effects of IL-26 utilizing both allo- and xeno-GVHD models and found that IL-26 markedly increases neutrophil levels and augments Th17 response associated with enhanced levels of G-CSF, IL-1β, and IL-6 in both models.
In fact, several differences were found between the allo- and xeno-GVHD models. The number of donor T cells infiltrated in GVHD-target organs were not affected by IL-26 in the allo-GVHD model (Figure 2A), whereas administration of anti-IL-26 mAb significantly reduced donor T cell infiltration in the xeno-GVHD model (Figure 5A,B). In addition, IL-26-induced enhancement of mouse Ccl3, Cxcl1, and Cxcl2 expression and lung fibroproliferation was prominently observed only in the xeno-GVHD model but not the allo-GVHD model (Figure 5G,H). These observed differences may be partially due to the higher expression levels of IL-26 in donor human CD4 T cells as compared with hIL-26Tg mouse-derived donor CD4 T cells (Figures 2D,G and 5E,F), and may be reflected in the survival curves shown for these two models (Figures 1A and 4A).
Our in vivo models indicate that IL-26 has the capacity to augment Th17 response (Figures 2D and 5E). Involvement of Th17 cells in the pathology of various autoimmune diseases has been reported.31 Regarding GVHD, transfer of in vitro polarized Th17 cells resulted in extensive pathologic cutaneous and pulmonary lesions in murine GVHD models.32 Our in vitro data suggest that the potential synergistic effect of IL-26 and nucleic acids (Figure 3A,B), or other inflammatory cytokines may lead to enhanced IL-1β and IL-6 expression in the GVHD-target organs (Figures 2I and 5G), resulting in a positive-feedback loop which further augmented Th17-cytokine production including IL-26, IL-17A, and IL-21 (Figure 8).
In the current study, we analyzed donor immune cell numbers and composition. In addition to the immune cells shown in Figure 2B, we also analyzed the percentage of NK cells, NKT cells, γδ T cells, plasma cells, basophils, eosinophils, mast cells, conventional, and plasmacytoid dendritic cells (data not shown). Among them, IL-26 appeared to be involved in the marked increase in neutrophil levels (Figures 1C and 2B). While neutrophils have a well-known role in host defense against pathogens, recent studies have described various effector functions of neutrophils.33 Although the role of neutrophils in the pathology of GVHD is not fully understood, production of reactive oxygen species and antigen presentation by neutrophils affected the severity of intestinal acute GVHD.34, 35 Moreover, release of NETs caused epitheliopathy and delayed epithelial wound healing in ocular chronic GVHD.36 In a human CD4 T cell-induced cutaneous xeno-GVHD model, murine neutrophils appeared to be involved in the onset of alopecia.37 Neutrophils are able to acquire different functions depending on the microenvironment and their differentiation/activation status.38 Our current work shows that IL-26 exposure was associated with elevated IL-6 and G-CSF expression (Figures 2I and 5G). Stimulation of human neutrophils with G-CSF enhanced antibody-dependent cellular cytotoxicity and cytokine production.39 Others reported that costimulation of mouse neutrophils with IL-6 and G-CSF increased matrix metalloproteinase-9.40 Therefore, in-depth analyses of the effector functions of neutrophils that accumulated in the GVHD-target organs in the presence of IL-26, as well as their involvement in the pathology of GVHD in our models, are needed in future studies.
G-CSF can be produced from diverse cell types such as monocytes/macrophages, epithelial cells, endothelial cells, and fibroblasts in response to various stimuli including IL-1β, IL-6, TNF-α, IL-17A, and lipopolysaccharide,41-44 suggesting that the overall IL-26-mediated positive-feedback loop is possibly associated with enhanced G-CSF expression. Likewise, although TGF-β1 plays a central role in fibrosis, diverse factors such as IL-17A, IL-11, IL-13, IL-1β, TNF-α, PDGF-α, CTGF also participate in the complex cellular mechanisms involved in fibrosis.45-47 Our group previously showed that IL-26 directly acted on fibroblasts to enhance collagen production,24 while alteration in the immune cell infiltration process and cytokine milieu by IL-26 is likely associated with the IL-26-mediated fibrosis. The mechanisms involved in IL-26 regulation of myofibroblast biology and collagen production will be evaluated in future studies.
In summary, our current work indicates that anti-IL-26 mAb treatment significantly suppressed the progression of GVHD via multiple mechanisms of action. We plan to analyze the serum concentration of IL-26 in GVHD patients and investigate the relationship between serum IL-26 levels and clinical outcomes for patients such as the severity or disease types of GVHD, types of hematological malignancies, and effects of steroid or other immunosuppressive agents. These basic findings will provide the necessary foundation for the use of humanized anti-IL-26 mAb for the treatment of chronic GVHD.
ACKNOWLEDGMENTS
This study was supported in part by a grant of the Ministry of Health, Labour, and Welfare, Japan (Grant Number 180101-01 (C.M.) and 210901-02 (C.M.)), JSPS KAKENHI Grant Numbers JP20K07683 (R.H.), JP21K16389 (T.I.), JP20H03471 (C.M.), and JP18H02782 (K.O.). This work was also supported by a research grant from the Japanese Society of Hematology (R.H.), a research grant from the Japan Research Institute of Industrial Science (R.H.), and President's Grant for Educational Excellence, Juntendo University Grant Number GP21-04 (K.O.). The authors thank Dr. Naoko Arano and Dr. Hitoshi Sasano for their excellent assistance with pulmonary function tests (Department of Respiratory Medicine, Juntendo University, Japan). The authors also thank members of the Atopy (Allergy) Research Center (Juntendo University Graduate School of Medicine, Japan) and members of the Laboratory of Morphology and Image Analysis, the Laboratory of Cell Biology, the Laboratory of Proteomics and Biomolecular Science, and the Laboratory of Molecular and Biochemical Research (Biomedical Research Core Facilities, Juntendo University Graduate School of Medicine, Japan) for their technical assistance and for the use of the experimental apparatus.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Ryo Hatano, Takumi Itoh, Yutaro Kaneko, Chikao Morimoto, and Kei Ohnuma are the inventors and patent holders of murine and humanized anti-human IL-26 mAbs. Yutaro Kaneko is the chief executive officer of Y's AC Co., Ltd, and Chikao Morimoto, Kei Ohnuma, Nam H. Dang, and Taketo Yamada are stockholders of Y's AC Co., Ltd. Other authors declare no competing financial interests associated with this manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the supporting information files of this article.




