Association of polymorphisms in genes coding for antioxidant enzymes and human male infertility
Abstract
Purpose
Although oxidative stress is thought to be an important cause of male infertility, primarily due to DNA and cell membrane damage, little is known about the genetic causes underlying suboptimal function of the seminal enzymatic antioxidant system. The aim of this study was to investigate the relationship of four potentially functional polymorphisms associated with oxidative stress pathway genes (superoxide dismutase—SOD2 lle58Thr and SOD2 rs4880, catalase—CAT C-262T, glutathione peroxidase 1—GPX1 Pro200Leu) and two null variants of the glutathione S transferase (GSTT and GSTM) genes and infertility risk.
Methods
A case control study was conducted on 313 infertile patients and 80 fertile donors. Each ejaculate was subjected to a seminal analysis that included the classical parameters seminal volume, sperm concentration, sperm motility, and sperm morphology, as well as sperm DNA fragmentation (patients only). Polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) and PCR multiplex methods were carried out for genotyping.
Results
Statistically significant differences were found between fertile donors and infertile patients for SNP CAT C-262T; the CC genotype was related with a twofold increased risk of infertility (odds ratio [OR] = 2.262; 95% confidence interval [CI] = 1.369–3.733; P = 0.001), whereas the CT genotype was associated with a protective effect (OR = 0.401; 95% CI = 0.241–0.667; P = 0.001). Surprisingly, the SOD2 Ile58ssThr SNP was not represented in the sample population, so its frequency in the current population frequenting fertility clinics in Madrid may be very low.
Conclusions
Our results suggest that the CAT SNP C-262T is potentially associated with an increased risk of male infertility.
1 INTRODUCTION
Reactive oxygen species (ROS) are free radicals having at least one unpaired electron and include molecules such the hydroxyl ion (OH), superoxide ion (O2), peroxyl radical (RO2), or hydrogen peroxide (H2O2). ROS originate from cellular reactions as by-products of the metabolism of oxygen and are normally neutralized by the homeostatic antioxidant systems. The ROS present in seminal plasma can originate from both endogenous or exogenous sources; endogenous sources may be derived from a high incidence of leukocytes in the seminal plasma and an overabundance of immature spermatozoa (sperm cells with residual cytoplasm) in the ejaculate of patients with varicocele (Agarwal, Makker, & Sharma, 2008; Allamaneni, Naughton, Sharma, Thomas, & Agarwal, 2004; Gomez et al., 1996). ROS in seminal plasma may also originate from exogenous sources such as exposure to ionizing radiation, cytotoxins, or poor lifestyle choice, including cigarette smoking or excessive alcohol consumption (Agarwal, Virk, Ong, & du Plessis, 2014).
Although the presence of ROS in seminal plasma is normally balanced by homeostatic antioxidant systems that facilitate an appropriate level of ROS required for normal physiological processes such as sperm capacitation, hyperactivation, acrosome reaction, and sperm–oocyte fusion, in some circumstances, unbalanced REDOX potential (a measure of oxidative stress defined as all known and unknown contributors of oxidative stress and not limited to a specific constituent as is the case in ROS or Total Antioxidant Capacity (TAC) assays) may arise due to an elevated production of ROS that is beyond the capacity of the animal's inherent antioxidant systems to cope with. Under such circumstances, high levels of ROS in the seminal plasma have the potential to not only cause sperm DNA damage, but also lipid peroxidation, sperm motility, and membrane fluidity reduction and apoptosis (Aitken, Gibb, Baker, Drevet, & Gharagozloo, 2016; Aitken, Smith, Jobling, Baker, & De Iuliis, 2014).
The enzymatic antioxidant systems in seminal plasma are a group of enzymes that include manganese superoxide dismutase (SOD2), catalase (CAT), glutathione peroxidase 1 (GXP1), and glutathione S transferase (GST) (O´Flaherty, 2014). SOD2 is an enzyme that catalyzes the detoxification of superoxide radicals in the mitochondrion (Ruiz-Sanz, Aurrekoetxea, Matorras, & Ruiz-Larrea, 2011; Yan et al., 2014). CAT has the capacity to detoxify H2O2 by converting it into H2O and O2 (Sabouhi, Salehi, Bahadori, & Mahdavi, 2014; Tefik et al., 2013). The GXP1 enzyme is related to the final electron transporter and neutralizes peroxide radicals into H2O (Suzen et al., 2010), whereas GST conjugates toxic electrophiles and other intermediates, with glutathione negating their toxicity (Olshan et al., 2010).
Although these enzymes appear to be conserved phylogenetically, species and individual specific polymorphisms may still produce changes in regard to their respective activities and, therefore, may be useful in understanding the underlying origins of idiopathic infertility. Hence, the aim of the current study was to elucidate and establish a genetic basis to investigate the relationship of enzymes associated with antioxidant system failure, conventional parameters of the WHO accredited sperm analysis, and male infertility. To examine this hypothesis, we compared the incidence of six potentially functional polymorphisms thought to be linked to oxidative stress pathway genes (SOD2, CAT, GXP1, GSTM, and GSTT) in infertile patients with that of donor controls of proven fertility attending a Spanish infertility clinic.
2 MATERIALS AND METHODS
2.1 Subjects and sample collection
The study was approved by the Bioethics Committee of the Universidad Autónoma de Madrid (CEI 60-1058) and informed consent was taken from all participants. Semen samples from a total of 393 subjects (patients and donors) attending a Spanish Fertility Clinic were analyzed consisting of 313 infertile patients undergoing infertility IVF/ICSI treatment and 80 donors with proven fertility who had already fathered at least one child. The patient cohort consisted of individuals that showed no evidence of any other fertility-related diseases, such as prostate cancer, cryptorchidism, varicocele, diabetes, seminal infections, or karyotype abnormalities. Each male donated 1 ml (patient cohort) or 0.5 ml (donor cohort) of semen that was obtained by masturbation after at least 4 days of abstinence; this abstinence period was based on the standard protocol used in the collaborating infertility clinic.
2.2 Seminal analysis
Each ejaculate was subjected to a classical seminal analysis approximately 30 min after liquefaction based on the recommendations and semen evaluation protocols and standards of the World Health Organization (2010); parameters analyzed included seminal volume, sperm concentration, sperm motility, and sperm morphology.
2.3 Sperm DNA fragmentation
Sperm DNA fragmentation (SDF) was assessed for all the samples in the patient cohort using the Sperm Chromatin Dispersion assay (SCD; Halosperm Kit, Halotech DNA, Madrid, Spain) according to the manufacturer's instructions. Processed slides were stained with SYBR-Green (SYBR-Green 10000x, cat. no. S7563; Thermo Fisher Scientific, Brawnschweig, Germany), mounted with Vectashield (Vectashield Mounting Medium, cat. no. H-1000; Vector Laboratories, Burlingame, CA) and observed with epifluorescence microscopy (Leica DMRB, Leica-Mycrosystems, Wetzlar, Germany).
2.4 DNA extraction
DNA extraction was performed using the phenol-chloroform method with proteinase-K treatment. Briefly, 500 μl of liquefied semen was centrifuged at 1800 rpm and the pellet resuspended in 100 μl of seminal plasma. This sample was then incubated overnight in a solution consisting of 8 μl 10 mg ml−1 proteinase K (Proteinase K Recombinant PCR Grade, cat. no. 03 115 887 001; Roche, Mannheim, Germany), 8 μl of 1 M DTT, and 100 μl extraction buffer (20 mM Tris-Cl, 20 mM EDTA, 200 mM NaCl, 4% SDS). Thereafter, 216 μl of phenol (phenol/chloroform/isoamyl alcohol 25:24:1, cat. no. 327115000; Acros Organics, Morris Plains, NJ) was added to the semen sample and the mixture agitated on a mechanical shaker for 2 min after which it was centrifuged at 8000 rpm for 10 min. The upper aqueous phase was then recovered and the same operation performed with 200 μl of 24:1 chloroform:isoamil (Chloroform reagent grade—cat. no. CL02031000; Scharlab, Spain; isoamylalkohol >98%—cat. no. 818969, Merck, Munich, Germany). The upper aqueous phase was then recovered once more to which 250 μl of cold (–20°C) 100% ethanol was also added. This solution was gently shaken until the DNA precipitated and the sample left to stand overnight at –20°C. The DNA was then washed with cold (–20°C) 70% ethanol and air-dried. To eliminate contaminating RNA, the pellet was resuspended in 50 μl of TRIS-EDTA buffer with 0.5 μl of 1 mg ml−1 RNAse (RNase DNase free, cat. no. 11 119 915 001; Roche, Mannheim, Germany) and incubated for 2 h at 37°C. Finally, the concentration of the extracted DNA was determined using a NanoDrop ND-1000 (Thermo Scientific, Brawnschweig, Germany) before diluting to a concentration of 50 ng μl−1 and stored at 4°C.
2.5 Genotype analysis
SOD2 Ile58Thr, SOD2 Val16Ala (rs4880), CAT C-262T (rs1001179), and GXP1 Pro200Leu (rs1050450) genotypes were determined using the polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) method. PCR products were then digested with the restriction enzymes EcoRV, HaeIII, SmaI, and HaeIII, respectively (FastDigest Restriction Enzymes, cat. nos. FD0303, FD 0154, and FD0664; Thermo Scientific). The respective primer sequences and the restriction enzymes are shown in Table 1. GSTM1-GSTT1 genotyping was performed using a PCR multiplex protocol (Arand et al. 1996). Briefly, three sets of primers where used in a unique PCR mix: forward 5′-GAACTCCCTGAAAAGCTAAAGC-3′ and reverse 5′-GTTGGGCTCAAATATACGGTTT-3′ for GSTM1, forward 5′-TTCCTTACTGGTCCTCACATCTC-3′ and reverse 5′-TCACCGGATCATGGCCAGCA-3′ for GSTT1 and forward 5′-GCCCTCTGCTAACAAGTCCTAC-3′ and reverse 5′-GCCCTAAAAAGAAAATCGCCAATC-3′ for albumin, as an internal control. PCR product sizes were 210, 480, and 350 bp, respectively.
| SNP | Primer PCR | Annealing Temperature | PCR product | Restriction enzyme | Restriction fragments |
|---|---|---|---|---|---|
| SOD2 Ile58Thr |
|
56 (°C) | 140 (bp) | EcoRV |
|
|
|
59 | 110 | HaeIII |
|
| CAT C-262T |
|
59 | 129 | SmaI |
|
|
|
57 | 338 | HaeIII |
|
2.6 Statistical analysis
Statistical analysis was performed using the SPSS 22 software for Windows (SPSS Inc., Chicago, IL). Associations between categorical variables (genotype frequencies and odds ratios [ORs]) were assessed by a χ2 test. Continuous variables (seminal parameters) were compared between groups using nonparametric tests (Mann–Whitney U-test —comparison between two groups and Kruskal–Wallis—comparison between more than two groups). Results were expressed as OR with 95% confidence interval (CI). A P value of <0.05 was considered statistically significant.
3 RESULTS
The demographic and clinical profiles of the patient and donor cohorts analyzed in this study are shown in Table 2; as expected, significant differences between both cohorts were obtained for almost all seminal parameters with the donor cohort showing the best seminal quality.
| Patients | Donors | P-value | |
|---|---|---|---|
| Age (year) | 37.81 ± 5.05 | 24.76 ± 4.96 | <0.001 |
| Volume (ml) | 3.33 ± 1.36 | 3.19 ± 1.19 | 0.431 |
| Sperm Concentration (× 106 ml−1) | 26.93 ± 18.41 | 53.82 ± 18.92 | <0.001 |
| Motility (%) | 38.93 ± 12.22 | 54.55 ± 6.36 | <0.001 |
| Morphology (%) | 3.41 ± 1.87 | 6.41 ± 1.71 | <0.001 |
| DNA fragmentation (%) | 19.69 ± 13.15 | No data | No data |
- P-value calculated with the Mann–Whitney U-test.
Genotype frequencies of each of the polymorphisms in the patient and donor cohort, along with the statistical level of significance, are shown in Table 3. Polymorphisms were observed for all SNPs but in the case of the SOD2 Ile58Thr polymorphism, only dominant homozygotes (Thr/Thr) were found. A significant difference in genotype frequency between the cohorts was only observed for the CAT C-262T SNP. The CC genotype was less frequent in the donor cohort (50%) compared to that of the patient group (69%), while the frequency of the CT genotype was almost double in the donor cohort (47%) compared to that found in the patient cohort (26%) (P = 0.002).
| Patients | Donors | ||||
|---|---|---|---|---|---|
| Genotype | number (observed) | Genotype frequency | number (observed) | Genotype frequency | P-value |
| Sod2 Ile58Thr | |||||
| Thr/Thr | 313 | 1.00 | 80 | 1.00 | |
| Ile/Thr | 0 | 0.00 | 0 | 0.00 | 0 |
| Ile/Ile | 0 | 0.00 | 0 | 0.00 | |
| Sod2 rs4880 | |||||
| Ala/Ala | 69 | 0.22 | 12 | 0.15 | |
| Ala/Val | 150 | 0.48 | 45 | 0.56 | 0.329 |
| Val/Val | 94 | 0.30 | 23 | 0.29 | |
| CAT C-262T | |||||
| CC | 215 | 0.69 | 40 | 0.50 | |
| CT | 82 | 0.26 | 37 | 0.47 | 0.002 |
| TT | 16 | 0.05 | 3 | 0.03 | |
| GPX1 Pro200Leu | |||||
| Pro/Pro | 139 | 0.44 | 31 | 0.39 | |
| Pro/Leu | 138 | 0.44 | 38 | 0.47 | 0.669 |
| Leu/Leu | 16 | 0.12 | 11 | 0.14 | |
| GSTT | |||||
| Present | 246 | 0.79 | 63 | 0.78 | |
| Absent | 67 | 0.21 | 17 | 0.22 | 0.975 |
| GSTM | |||||
| Present | 151 | 0.48 | 43 | 0.53 | |
| Absent | 162 | 0.52 | 37 | 0.47 | 0.420 |
- P-value calculated by χ2 test.
The OR of being infertile for each genotype was calculated against the other two genotypic options. A significant OR was only noted in CAT CC and CT genotypes (Figure 1); in fact, the CC genotype was associated with a twofold increased risk of infertility (OR = 2.262; 95% CI = 1.369–3.733; P = 0.001), whereas the CT genotype was associated with a protective effect (OR = 0.401; 95% CI = 0.241–0.667; P = 0.001).
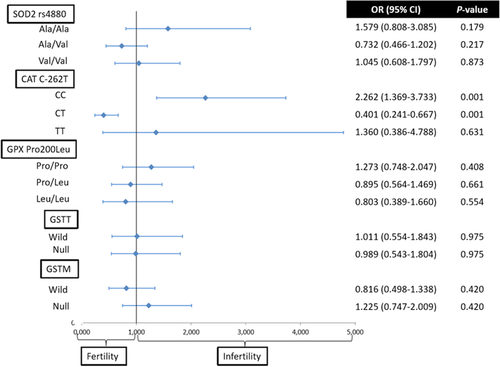
Median differences in classical seminal parameters (seminal volume, sperm concentration, % sperm motility, % sperm morphology, and % SDF) were compared between patients and donors for each of the genotypes (Table 4). The results of the analysis revealed significant differences for only the CAT SNP with respect to motility (P = 0.047) and morphology (P = 0.024). Pairwise comparisons for these parameters also revealed significant differences for morphology between the CC and CT genotypes (H = –32.187, adjusted P = 0.029, r = –0.130). Moreover, analysis of the median values for sperm concentration between patients and donors for the SNP CAT C-262T genotypes was close to being significant (P = 0.051).
| Genotypes | P-value1 | P-value2 | |||||
|---|---|---|---|---|---|---|---|
| SOD2rs4880 | Ala/Ala | Ala/Val | Val/Val | ||||
| Volume | Patients | 3.25 | 3.43 | 3.21 | 0.749 | 0.781 | |
| Donors | 3.08 | 3.27 | 3.08 | 0.722 | |||
| Concentration | Patients | 26.19 | 26.83 | 27.47 | 0.983 | 0.736 | |
| Donors | 51.50 | 54.56 | 53.60 | 0.984 | |||
| Motility | Patients | 37.04 | 39.85 | 38.54 | 0.354 | 0.108 | |
| Donors | 55.83 | 54.77 | 53.47 | 0.567 | |||
| Morphology | Patients | 3.34 | 3.43 | 3.39 | 0.955 | 0.526 | |
| Donors | 6.41 | 6.59 | 6.04 | 0.768 | |||
| DNA fragmentation | Patients | 21.06 | 19.31 | 19.32 | 0.634 | 0.634 | |
| Donors | |||||||
| CAT C-262T | CC | CT | TT | ||||
| Volume | Patients | 3.37 | 3.16 | 3.53 | 0.504 | 0.820 | |
| Donors | 3.07 | 3.36 | 2.60 | 0.487 | |||
| Concentration | Patients | 26.26 | 27.51 | 33.02 | 0.419 | 0.051 | |
| Donors | 52.93 | 53.94 | 64.00 | 0.294 | |||
| Motility | Patients | 38.48 | 38.72 | 45.93 | 0.080 | 0.047 | |
| Donors | 55.25 | 54.18 | 50.00 | 0.171 | |||
| Morphology | Patients | 3.31 | 3.54 | 4.12 | 0.159 | 0.024 | |
| Donors | 6.48 | 6.48 | 5.00 | 0.088 | |||
| DNA fragmentation | Patients | 19.84 | 18.66 | 22.88 | 0.849 | 0.849 | |
| Donors | |||||||
| GPX1 Pro200Leu | Pro/Pro | Pro/Leu | Leu/Leu | ||||
| Volume | Patients | 3.31 | 3.49 | 2.79 | 0.018 | 0.044 | |
| Donors | 3.20 | 3.20 | 3.11 | 0.983 | |||
| Concentration | Patients | 27.92 | 25.37 | 29.05 | 0.780 | 0.701 | |
| Donors | 53.19 | 54.05 | 54.81 | 0.789 | |||
| Motility | Patients | 38.64 | 38.88 | 39.44 | 0.830 | 0.783 | |
| Donors | 55.64 | 54.05 | 53.18 | 0.642 | |||
| Morphology | Patients | 3.41 | 3.31 | 3.75 | 0.597 | 0.618 | |
| Donors | 6.25 | 6.59 | 6.18 | 0.866 | |||
| DNA fragmentation | Patients | 20.88 | 19.06 | 17.64 | 0.509 | 0.509 | |
| Donors | |||||||
| GSTT | Wild (+) | Nul (–) | |||||
| Volume | Patients | 3.33 | 3.33 | 0.651 | 0.889 | ||
| Donors | 3.25 | 2.95 | 0.508 | ||||
| Concentration | Patients | 27.50 | 24.85 | 0.569 | 0.604 | ||
| Donors | 53.46 | 58.76 | 0.620 | ||||
| Motility | Patients | 39.00 | 38.65 | 0.921 | 0.859 | ||
| Donors | 54.91 | 54.91 | 0.340 | ||||
| Morphology | Patients | 3.42 | 3.35 | 0.855 | 0.986 | ||
| Donors | 6.21 | 6.21 | 0.460 | ||||
| DNA fragmentation | Patients | 19.65 | 19.82 | 0.923 | 0.923 | ||
| Donors | |||||||
| GSTM | Wild (+) | Null (–) | |||||
| Volume | Patients | 3.32 | 3.34 | 0.464 | 0.719 | ||
| Donors | 3.30 | 3.05 | 0.460 | ||||
| Concentration | Patients | 27.25 | 26.64 | 0.704 | 0.460 | ||
| Donors | 53.14 | 54.59 | 0.961 | ||||
| Motility | Patients | 39.93 | 38.00 | 0.365 | 0.266 | ||
| Donors | 53.81 | 55.40 | 0.152 | ||||
| Morphology | Patients | 3.46 | 3.36 | 0.455 | 0.256 | ||
| Donors | 6.54 | 6.24 | 0.556 | ||||
| DNA fragmentation | Patients | 19.33 | 20.03 | 0.819 | 0.819 | ||
| Donors | |||||||
- The data shown are the mean for volume (ml), concentration (106 ml−1), motility (%), morphology (%), and DNA fragmentation (%). P-value1 = comparison between genotypes from patients and donors, P-value2 = comparison between genotypes for all 393 samples. Statistical analysis—the Kruskal–Wallis test was used to compare for volume, sperm % motility, % morphology, and % DNA fragmentation for three genotypes, whereas the Mann–Whitney U-test was used to analyze data when two genotypes were compared.
4 DISCUSSION
This study explored the association of six polymorphisms of antioxidant enzymes (SOD2.lle58Thr, SOD2.rs4880, CAT C-262T, GPX1 Pro200Leu, GSTT, and GSTM) with observed infertility in patients from a Spanish fertility clinic; a statistically significant association was observed between the SNP for CAT C-262T and infertility. The CT genotype was associated with a protective or beneficial effect with respect to fertility.
For the purposes of this study, we have assumed that donors and patients attending the clinic were representative of the current Spanish cosmopolitan population as the clinic is located in Madrid (Spain); we therefore compared the genotype frequencies of the Madrid population with that obtained for the same polymorphisms in other European populations (Table 5) (Brans, Lyrenäs, de Faire, & Morgenstern, 2005; Kowalski et al., 2010; Suzen et al., 2010; Xiong, Chen, Ding, Zhang, & Zhang, 2015). We observed that for SNP GPX1 Pro200Leu and for the variants of the GST enzymes our frequencies were within or close to within the frequency limits obtained for the same polymorphisms of other European populations. For the SNP SOD2 rs4880, the only differing frequency was for the heterozygote genotype (0.56 vs. 0.28–0.47). In contrast, the SNP CAT C-262T was the one with the largest differences for all genotype options, while for SNP SOD2 Ile58Thr, we could only find the dominant homozygote genotype in the current population.
| Dominant homozygote | Heterozygote | Recessive homozygote | |
|---|---|---|---|
| SOD2 Ile58Thr | 0.98–1 | 0.01–0.02 | 0.00 |
| SOD2 rs4880 | 0.06–0.54 | 0.22–0.47 | 0.08–0.52 |
| CAT C-262T | 0.57–0.63 | 0.30–0.37 | 0.03–0.05 |
| CAT Pro200Leu | 0.41–0.59 | 0.35–0.45 | 0.06–0.15 |
| GSTT | 0.13–0.26 | ||
| GSTM | 0.42–0.60 |
The CAT enzyme, coded in chromosome 11p3, is the main enzyme in the detoxification of H2O2 to H2O. H2O2 plays an important role in male infertility as it causes lipid peroxidation, which reduces the fluidity of the membrane, thus causing a reduction in motility and poor oocyte–sperm membrane fusion (Kemal Duru, Morshedi, & Oehninger, 2000; Oehninger, Blackmore, Mahony, & Hodgen, 1995). H2O2 in seminal plasma can appear directly as a consequence of the activity of leukocytes or indirectly as a product of the detoxification of ROS by superoxide dismutase. It has been shown that seminal plasma of infertile patients has a higher H2O2 concentration and a lower CAT activity than that of healthy controls (Zelen, Mitrović, Jurisic-Skevin, & Arsenijević, 2010). Recent studies have also demonstrated that an external supplementation with CAT when cryopreserving semen improves postthaw sperm motility and sperm viability (Moubasher, El Din, Ali, El-sherif, & Gaber, 2013).
In the current study, we found significant differences in the genotype frequencies for SNP CAT C-262T between patients and fertile donors. Specifically, we observed an increased frequency (almost double) of the CT genotype in donors when compared with patients. Thus, we hypothesize that the T allele may be a variant of the enzyme with a higher transcriptional rate and be beneficial in heterozygosity. This observation is concordant with the studies of Sabouhi et al. (2014) and Forsberg, Lyrenäs, de Faire and Morgenstern (2001), both of whom observed the T allele to be related to higher CAT levels. In contrast, Tefik et al. (2013) and Ahn et al. (2006) showed a lower enzyme activity for the CT and TT genotypes of the CAT C-262T polymorphism; it is possible that these differences may be a consequence of the particular characteristics of the populations included in each study. According to Suzen et al. (Suzen et al. 2010), the CAT C-262T polymorphism shows important differences in the genotype frequencies between populations. Moreover, when we compared the values of the main parameters of the traditional sperm analysis between genotypes, we observed that the highest quality values for all parameters appeared in the genotypes containing the T allele. Significant differences between genotypes were also obtained for motility and morphology, although pairwise comparisons only revealed significant differences for morphology between the CT and CC genotypes, with CT genotype being associated with better semen quality; close to significant differences were also apparent for the sperm concentration parameter. Moreover, for SDF, the highest sperm quality was also found associated with the CT genotype. Finally, it was noted that the occurrence of infertility was 2.26 times more likely to be associated with the CC genotype, than for the other two genotypes, while the CT genotype showed a protective effect; these observations support the hypothesis that the CT genotype could be beneficial in terms of protection against oxidative stress.
Manganese superoxide dismutase 2 (SOD2) is a mitochondrial antioxidant enzyme coded in chromosome 6q25. It catalyzes the detoxification of superoxide radicals in the mitochondrion producing H2O2 and O2 as by-products. In the SOD2 rs4880 polymorphism, there is a change of Ala to Val in position -9 of the N-terminal mitochondrial targeting sequence, which leads to a variant of the enzyme that is less efficiently transported to the mitochondrion (Hiroi et al., 1999). The results of our study were not consistent with this hypothesis as we observed a slightly higher representation of the Val allele in fertile donors than in patients. However, our results are concordant with the observations of Yan et al. (2014) that found a higher presence of the Val allele in fertile males; they suggested that the higher efficient Ala allele should produce higher levels of H2O2, which may be detrimental for sperm quality. Moreover, we noted that Ala/Val and Val/Val genotypes were associated with higher quality seminal parameters, although the differences between these genotypes were low; in addition, the Ala/Ala genotype showed a 1.6 times higher OR of being infertile than the other two genotypes.
The SOD2 Ile58Thr polymorphism affects the stability of the protein (Tefik et al., 2013). Specifically, Borgstahl et al. (1996) demonstrated that the Thr variant has a thermal stability between 15 and 20°C lower that the Ile variant, which deeply compromises its viability in vivo. In our study, we could not find a representation of that polymorphism in our population as we only found the Ile/Ile genotype both in patients and in donors; this observation would suggest that the Ile/Ile genotype is overrepresented in our studied population and may be explained based on the conclusions obtained by Borgstahl et al. (1996)
The GPX1 enzyme, coded in chromosome 3p21, is a selenium-dependent peroxidase and is ubiquitously expressed in humans. It works as the final electron transporter that neutralizes peroxide radicals to water and oxygen (Hong, Tian, & Zhang, 2013). In the Pro200Leu polymorphism, there is a substitution of Pro to Leu at codon 200 located in the c-terminal region of the protein. Najafi, Ghasemi, Roustazadeh and Farajollahi (2014) in a computer predictor study concluded that this polymorphism is located on the protein surface in a nonfunctional region and that the substitution does not affect protein stability or structure. However, Hu and Diamond (2003) concluded that the Leu allele is less effective than the Pro allele when neutralizing peroxide radicals; in contrast with this observation, our results showed a higher presence of the Leu allele in fertile donors than in patients and better values for almost all the parameters in the traditional sperm analysis in the Leu/Leu genotype group (the only parameter differing was the volume). Curiously, we also found significant differences between genotypes for the parameter volume, both in the patient population and in the global analysis. The OR analysis for infertility showed that the ratio of being infertile was almost similar for the Pro/Leu and Leu/Leu genotypes and was 1.27 times higher for the Pro/Pro genotype than for the other two.
GST comprises a family of enzymes that conjugate toxic intermediates with glutathione inactivating them and facilitating their excretion. GSTM (chromosome 1p13.3) and GSTT (chromosome 22q11.2) are two human GST isozymes that can present a null allele as a result of a deletion. Several studies have been carried out recently to try and clarify the relationship between the null variants and male infertility but this relationship is still controversial. For the GSTM null genotype, some studies have concluded that it is associated with a higher risk of male infertility (Roshdy, Hussein, Zakaria, & Sabry, 2015; Tirumala Vani et al., 2010) but others show no relationship at all (Olshan et al., 2010). Similarly, although several studies have shown a relationship between the GSTT null genotype and male infertility (Wu et al., 2013), there are others that reveal no relationship or even a beneficial effect of the null genotype with respect to male infertility (Olshan et al., 2010). Moreover, some studies point out that the effects of both null genotypes may also be related to exposure to air pollution (Rubes, Selevan, Sram, Evenson, & Perreault, 2007). In our study, we observed almost similar frequencies and an OR of infertility for both patients and donors with respect GSTT, whereas the GSTM null genotype showed a slightly higher frequency in patients than in donors and an OR indicating that the occurrence of infertility was 1.25 times more likely than for the wild variant. Consequently, our findings are concordant with the most accepted hypothesis that both null variants are detrimental for male infertility.
We are aware that our study has both strengths and weaknesses. The first strength is the relatively high number of individuals included in the study; second, all subjects included in the donors’ cohort had proven fertility as they had already fathered at least one child. With respect to the study's weaknesses, although we know that there is a difference in the mean age of the two cohorts included in the study, all males included in the study were young men within the well-recognized fertility window and we are confident that this difference is not confounding our results as age does not influence genotypic frequencies. In addition, although seminal parameters are influenced by patient exposure factors such as BMI, smoking, and diet, these aspects are difficult or virtually impossible to fully define in the individual patients and thus we have not considered them in our study.
In conclusion, we found statistically significant differences for SNP CAT C-262T between fertile donors and infertile patients, with the CT genotype showing a potentially protective role. However, we also found interesting associations for the other polymorphisms we examined that could help us to understand the roles of the antioxidant pathways in ROS-related male infertility. According to our observations, we predict that males with the Val allele in the SOD2 gene, the Leu allele in the GPX1 gene, and GSTs with wild variants, should have a better response to increased ROS. We are confident that more research regarding the relationship between gene polymorphisms associated with seminal plasma antioxidant capacity will lead to a better understanding of male factor infertility and could therefore be applied to improve both patient diagnosis and donor selection with genetic background testing.
ACKNOWLEDGMENTS
The Ministry of Economy, Industry and Competitiveness of Spain supported this study.
AUTHORS CONTRIBUTIONS
Study design was performed by J.G. and R.R.; data collection was carried out by A.G. and M.C.; data analysis was done by A.G.; A.G. and S.J. were involved in the manuscript preparation; J.G. and R.R. were manuscript supervision.




