Bronchiolar adenoma/ciliated muconodular papillary tumor mixed with adenocarcinoma in situ in the same tumor
Abstract
Bronchiolar adenoma (BA)/ciliated muconodular papillary tumor (CMPT) is defined as a benign tumor composed of epithelial and basal cells. Recently, some cases with driver mutations or malignant transformation have been observed. Thus, whether BA/CMPT is benign or malignant remains controversial. We herein report an extremely rare case of a 68-year-old woman with a CMPT accompanied by adenocarcinoma in situ (AIS). BA/CMPT existed inside the AIS. The BA/CMPT component did not show any driver mutations; however, the AIS component had an EGFR driver mutation in exon 19. The accumulation of cases and further studies are needed to discuss the malignant potential of BA/CMPT.
INTRODUCTION
Bronchiolar adenoma (BA)/ciliated muconodular papillary tumor (CMPT) is defined as a benign tumor composed of epithelial and basal cells. Recently, some cases with driver mutations or malignant transformation have been observed.1-5 Thus, whether BA/CMPT is benign or malignant is considered controversial. We herein report an extremely rare case of a 68-year-old woman with a CMPT accompanied by adenocarcinoma in situ (AIS).
Case report
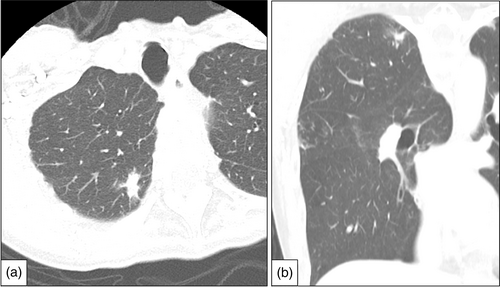
The patient was a 68-year-old woman who underwent surgery for left lower lobe lung adenocarcinoma. A right lung tumor was found on computed tomography (CT) before surgery to treat adenocarcinoma of the left lung; thus, she was readmitted for surgical treatment. Chest CT showed a part-solid nodule exhibiting a solid part of 13 mm in the right upper lobe (Figure 1). Therefore, partial resection of the right upper lung was performed.
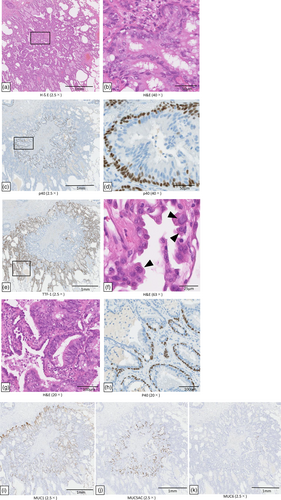
The postoperative pathological findings showed a mixture of AIS and BA/CMPT in the same tumor. The tumor components inside the AIS showed papillary growth (Figure 2a), and the basal cell layer was positive for p40 (Figure 2c), p63, and cytokeratin (CK) 5/6, but there was no nuclear atypia. Bilayering with basal cells was observed without disturbance of the arrangement of the basal cells. The luminal side showed epithelial columnar cells and goblet cells, which secreted mucous material (Figure 2b). These findings were characteristic of proximal BA (classical CMPT). Based on the above, the internal tumor was diagnosed as BA/CMPT. The tumor outside the BA/CMPT was positive for thyroid transcription factor-1 (TTF-1) (Figure 2e), and negative for p40 and p63. Hematoxylin and eosin (H&E) staining showed enlargement of the nuclei and the proliferation of alveolar epithelial replacement (Figure 2f). No invasion was observed. Based on above, the marginal tumor was diagnosed as AIS. Thus, BA/CMPT existed inside the AIS. Since the AIS and BA/CMPT were non-invasive, no alveolar wall destruction was observed, and the elastic fiber structure was preserved. The border zone between the BA/CMPT and AIS was a mixture of adenoma and adenocarcinoma components (i.e., where basal cells were present and absent) (Figure 2g,h). Both tumors were positive for mucin 1 (MUC1), while only the BA/CMPT component was positive for MUC5AC and MUC6 (Figure 2i–k). Genetic analysis using Oncomine Dx Target Test Multi-CDx (SRL Inc.) was performed by a next-generation sequencing method. Tissue macrodissection at the boundary between BA/CMPT (mainly proximal-type BA) and AIS was performed in our pathology department. An exon 19 driver mutation was detected in the AIS component alone. No driver mutations were detected in the BA/CMPT component (Table 1).
| BA/CMPT | AIS | |
|---|---|---|
| EGFR L858R | not detected | negative |
| EGFR Exon19 | not detected | positive |
| EGFR E709X | not detected | negative |
| EGFR g719X | not detected | negative |
| EGFR S768I | not detected | negative |
| EGFR L768I | not detected | negative |
| EGFR L861X | not detected | negative |
| EGFR T790M | not detected | negative |
| EGFR Exon20 | not detected | negative |
| BRAF | not detected | negative |
| ALK | negative | negative |
| ROS1 | negative | negative |
| RET | negative | negative |
| MET | negative | negative |
| NTRK | negative | negative |
| KRAS | not detected | negative |
| ERBB2 | not detected | negative |
DISCUSSION
BA/CMPT is considered to be a bilayered benign tumor composed of epithelial and basal cells. BA/CMPT is difficult to distinguish from invasive mucinous adenocarcinoma (IMA) or AIS by imaging; thus, a histopathological examination and observation of the cell arrangement are important for its diagnosis.6 Based on the composition of epithelial cells, it is divided into proximal-type BA and distal-type BA. Proximal-type BA is composed of a mixture of mucous cells and ciliated cells, and distal-type BA is composed of type II alveolar epithelium and club cells. However, mixed cases of proximal and distal types are common; thus, it should be considered as a continuous spectrum.7 In our case, both proximal- and distal-type BA were observed (Figure 3a,b). The boundaries between them were both bilayered with basal cells, but there was a mixture of areas with and without epithelial columnar cells and mucous cells. (Figure 3c,d). Distal-type BA is strongly positive for TTF-1, while proximal-type BA is partially positive or negative for TTF-1.7 According to previous studies, driver mutations or malignant transformation in BA/CMPT have been reported in several cases.1-5, 8-11 It is therefore controversial whether BA/CMPT is benign or malignant. In reported cases that showed malignant transformation, the lack of continuity of basal cells and the recognition of the same driver mutation were considered to be grounds for malignant transformation of BA/CMPT. In our case, BA/CMPT and AIS coexisted in the same tumor. Although there are some reports of malignant transformation of BA/CMPT into IMA,1, 2 there is only one other report of BA/CMPT and AIS in the same tumor.12 In our case, the tumor boundaries between BA/CMPT and AIS were relatively clear. In contrast, in the report by Zhao et al. the boundaries were unclear and there were many lesions with characteristics of adenomatous malignant transformation inside the tumor. Furthermore, there was no mention in their report as to whether the BA/CMPT had shown malignant transformation to AIS or whether the two tumors had separate origins and were incidentally mixed. To our knowledge, there is only one similar case of a well-defined tumor border, which was reported by Wang et al.5 Their case also showed BA/CMPT in the invasive adenocarcinoma. The difference between their report and ours is that the driver mutation in our case (exon 19 mutation) was only found in the AIS component, whereas the L858R mutation in their case was found in both the BA/CMPT and AIS components. Therefore, their case of invasive adenocarcinoma presumably involved malignant transformation of BA/CMPT.
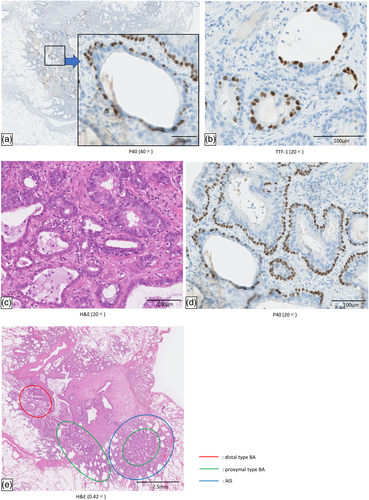
There are several possible origins of the AIS in our case. One is the malignant transformation of distal-type BA to AIS, because both consisted of nonmucus-producing cells. However, as shown in Figure 3e, the distal-type BA and AIS in our case were located some distance apart from each other. Another possibility is that proximal type BA showed malignant transformation to AIS because both cases were positive for MUC1. However, considering that MUC5AC and MUC6 were only detected in BA/CMPT, that the components showed different MUC expression patterns, and that exon 19 was only expressed in the AIS component, it is thought that the two tumors were incidentally mixed. However, this case report is associated with one important limitation, namely that the driver mutation in the BA/CMPT component could not be detected by genetic analysis due to insufficient DNA amplification.
The present case, in which the two tumor components were located adjacent to each other with clear boundaries is very rare. Further studies are needed including investigation of the nature and mechanism of malignant transformation of BA/CMPT.
In conclusion, the coexistence of BA/CMPT and AIS in the same tumor is extremely rare, and the accumulation of further cases is needed. The malignant potential of BA/CMPT should also be considered.
AUTHOR CONTRIBUTIONS
Suiha Uchiyama, Kenichi Mizutani and Masayuki Tanahashi contributed to the conception of the article and wrote the manuscript. Eriko Suzuki, Naoko Yoshii, Takuya Watanabe, Hiroyuki Tsuchida, Shogo Yobita, Kensuke Iguchi, Minori Nakamura, Takumi Endo, Seishiro Takahashi and Hiroshi Ogawa helped manuscript preparation and constructive discussions.
ACKNOWLEDGMENTS
We thank the staff of Pathology Diagnostic Department for their assistance in the diagnosis of the patient, and thank Japan Medical Communication for editing the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest in association with the present study.