The development of a tumor-associated autoantibodies panel to predict clinical outcomes for immune checkpoint inhibitor-based treatment in patients with advanced non-small-cell lung cancer
Jing Zhao, Yang Wu and Yuan Yue contributed equally to this work and should be considered as co-first authors.
Funding information: CAPTRA-Lung Research Funds, Grant/Award Number: CAPTRALung2021005; Chinese Academy of Medical Sciences, Grant/Award Number: CIFMS 2018-I2M-1-003; Innovation Fund
Abstract
Background
Immune checkpoint inhibitors (ICIs) have become one important therapeutic strategy for advanced non-small-cell lung cancer (NSCLC). It remains imperative to identify reliable and convenient biomarkers to predict both the efficacy and toxicity of immunotherapy, and tumor-associated autoantibodies (TAAbs) are recognized as one of the promising candidates for this.
Patients and Methods
This study enrolled 97 advanced NSCLC patients with ICI-based immunotherapy treatment, who were divided into a training cohort (n = 48) and a validation cohort (n = 49), and measured for the serum level of 35 TAAbs. According to the statistical association between the serum positivity and clinical outcome of each TAAb in the training cohort, a TAAb panel was developed to predict the progression-free survival (PFS), and further examined in the validation cohort and in different subgroups. Similarly, another TAAb panel was derived to predict the occurrence of immune-related adverse events (irAEs).
Results
In the training cohort, a 7-TAAb panel composed of p53, CAGE, MAGEA4, GAGE7, UTP14A, IMP2, and PSMC1 TAAbs was derived to predict PFS (median PFS [mPFS] 9.9 vs. 4.3 months, p = 0.043). The statistical association between the panel positivity and longer PFS was confirmed in the validation cohort (mPFS 11.1 vs. 4.8 months, p = 0.015) and in different subgroups of patients. Moreover, another 4-TAAb panel of BRCA2, MAGEA4, ZNF768, and PARP TAAbs was developed to predict the occurrence of irAEs, showing higher risk in panel-positive patients (71.43% vs. 28.91%, p = 0.0046).
Conclusions
Collectively, our study developed and validated two TAAb panels as valuable prognostic biomarkers for immunotherapy.
INTRODUCTION
Lung cancer is one of the most common types of cancer and accounts for the largest number of cancer-related deaths worldwide.1, 2 The high mortality rate is mainly due to the fact that lung cancer patients are often diagnosed in the advanced stage due to a lack of effective therapeutic methods.3 Non-small-cell lung cancer (NSCLC) is the major subtype of lung cancer and accounts for 80–85% of the total lung cancer.4 The recent development of immune checkpoint inhibitor (ICI)-based treatment with anti-PD-1 (programmed cell death protein 1), PD-L1 (programmed death-ligand 1) or CTLA-4 (cytotoxic T lymphocyte-associated protein-4) monoclonal antibodies has improved the survival of NSCLC patients,5 while the response rate of anti-PD-1/PD-L1 monotherapy for NSCLC was reported to be only approximately 15–20%.6-9 it is therefore necessary to identify valuable biomarkers to predict therapeutic efficiency and provide guidance for immunotherapy.
Some tumor tissue biomarkers have been described, such as tumor PD-L1 expression, tumor mutation burden, microsatellite instability, specific somatic mutations (STK11 and KEAP1 mutation), and intratumoral T cell infiltration,10-12 but due to limitations, including invasive sampling methods, difference in testing platforms and commercial kits, various cut-off values for immunotherapy agents, and the heterogeneous PD-L1 expression within tumors, these biomarkers are not convenient and reliable enough to be widely used in clinical practice. Taking advantage of noninvasive sampling, peripheral blood biomarkers have attracted attention. Many studies have suggested that the neutrophil-to-lymphocyte ratio, number of HLA (human leukocyte antigen)-DR monocytes, circulating PD-1+ CD8 (cluster of differentiation 8)+ T cell activity, and the number of NK (nature killer) cells in blood are related to the clinical outcome of ICI-based therapy.13-15 However, the predictive value of these biomarkers needs to be further verified and many other parameters in peripheral blood remain to be clarified to better understand the antitumor immune response.
Previous studies have uncovered a diversity of cancer tissue-derived cellular proteins that can be shed into the humoral system and trigger the immune system to generate tumor-associated autoantibodies (TAAbs) even before diagnosis.16-18 These proteins mainly include tumor-suppressor gene proteins such as p5319 and p16,20 messenger RNA (mRNA) binding proteins such as IGF2BP2,21 cell-cycle regulatory proteins such as Cyclin B1,22 and apoptosis inhibitors protein such as Survivin.23 Although the underlying mechanisms are not completely understood, the production of TAAbs can reflect higher immunologic reactivity and enhanced immune surveillance in cancer patients, and researchers have shown the application potential of TAAbs as serological biomarkers in early cancer diagnosis as well as a therapeutic response prediction for immunotherapy. Some studies have reported that the positivity of TAAbs such as NY-ESO-1, XAGE1, and SIX2 is indicative of better clinical responses to anti-PD1 monotherapy.24, 25 In addition to these preliminary findings, no serum TAAb-based strategy has been appoved to predict the effcacy of immunotherapy in clinic, which warrants more comprehensive characterization. In this study, we aimed to develop TAAb panels to clinically predict the efficacy and toxicity of ICI-based treatment in patients with advanced NSCLC.
MATERIALS AND METHODS
Patients
This study was a single-center retrospective study conducted in Peking Union Medical College Hospital (PUMCH). Patients who were diagnosed with advanced NSCLC in metastatic or unresectable stages between July 2017 and September 2020 were enrolled in the study. Inclusion criteria were (1) age more than 18 years, (2) confirmed NSCLC by pathology, (3) staged IIIB/IV according to the eighth edition of the TNM classification for lung cancer, and (4) received PD-1/-L1 inhibitor-based treatment. Exclusion criteria included (1) have autoimmune diseases, (2) received other immunotherapy including but not limited to vaccines and adoptive cellular immunotherapy, (3) active multiple primary malignancies, and (4) receiving intensive immunosuppressive agents. For all enrolled patients, clinical information was collected including gender, age, smoking, stage, pathology, and driver gene alterations (Supporting Information Table S1). Driver gene alterations include EGFR (Epidermal growth factor receptor) mutation, ALK (Anaplastic lymphoma kinase) fusion, RET (Ret proto-oncogene) fusion, and KRAS (Kirsten rat sarcoma viral oncogene homologue) G12C mutation.
Specimen collection
Ten milliliters of the peripheral blood of each patient was drawn into an EDTA (ethylenediamine tetraacetic acid)-anticoagulation tube one week before the initiation of ICI-based treatment. The blood was centrifuged at 300 g for 5 min at room temperature to obtain plasma. The plasma was stored at −80°C before TAAb measurement.
TAAb serum level measurement
Enzyme-linked immunosorbent assay (ELISA) was used to determine the reactivity of TAAbs. A total of 35 purified tumor-associated antigens (TAAs) (Annexin1, TTC14, MAGEA3, NY-CO-8, AKAP4, CFL1, NY-ESO-1, P53, GAGE7, PGP9.5, CAGE, MAGEA1, SOX2, GBU4-5, BRCA2, PSMC1, TARDBP, ZNF768, DCLK1, TPI1, PGAM1, AIF, PEBP1, HIP, UTP14A, TLN, RASSF7, PARP, XAGE1b, PIM1, Trim21, MAGEA4, IMP2, IMPDH1, and HSP105) were expressed in E. coli and purified via chromatography. The TAAs were then added to a 96-well plate (Thermo Scientific, #456537) pre-immobilized with bovine serum albumin (BSA)-biotin (Thermo Scientific, #29130). After incubation, patient plasma samples were added to the microwells. allowing the TAAbs to bind to their respective TAAs. Horseradish peroxidase-conjugated anti-human IgG and substrate 3,3′,5,5′-tetramethylbenzidine were then added for color development. Finally, the absorbance was read at optical density (OD) 450 nm on a spectrometer. The cutoff OD value for a positive result was calculated as the mean OD value of healthy control subjects plus twice the standard deviation (Supporting Information Table S2).
Therapeutic response evaluation
The primary endpoint in this study was progression-free survival (PFS) of ICI-based treatment, and the secondary endpoints were overall response rate (ORR) and the incidence of irAEs. Efficacy was evaluated according to RECIST v1.1,26 and ORR was defined as complete response plus partial response (PR). PFS was defined as the interval from the initiation of treatment to disease progression or death from any cause. irAEs were diagnosed by two experienced oncologists according to the CSCO guideline for the management of immunotherapy-related toxicity.27
Statistical analysis
Statistical analyses were performed using SPSS 22.0. Comparisons between groups were performed using the chi-square test or Fisher's exact test for categorical variables such as response rate and incidence of irAEs. PFS was estimated using the Kaplan–Meier method and compared by log rank test. For factors with p value <0.05 in the chi-square test or Fisher's exact test, they were further examined by binary or ordinal logistic regression to determine whether they were independent factors affecting outcome. p value <0.05 (two-tailed) was defined as statistical significance.
RESULTS
Characterization of the study population
A total of 97 patients with advanced NSCLC who received ICI-based treatment were enrolled into this study, including a discovery cohort (n = 48) and a validation cohort (n = 49). Baseline characteristics are summarized in Table 1. The TAAb positivity rates in the two groups were 72.9% and 73.9%, respectively. Forty-three patients with a driven gene mutation were also included, 20 (41.6%) into the discovery cohort and 23 (46.9%) into the validation cohort. Apart from a higher proportion of ICIs used as first-line treatment in the discovery group, the other clinical characteristics, such as age, gender, smoking history, ECOG score, pathology, stage, and treatment regimens, were well balanced between the two groups.
| Characteristics | Discovery cohort (n = 48) | Validation cohort (n = 49) | p value |
|---|---|---|---|
| Age, years | 0.87 | ||
| Median, range | 65 (48–78) | 65 (44–80) | |
| Gender | 0.318 | ||
| Male | 34 (70.8%) | 30 (61.2%) | |
| Female | 14 (29.2%) | 19 (38.7%) | |
| Smoking history | 0.186 | ||
| No | 20 (41.7%) | 27 (55.1%) | |
| Yes | 28 (58.3%) | 22 (44.9%) | |
| ECOG | 0.203 | ||
| 0–1 | 40 (83.3%) | 45 (91.8%) | |
| ≥2 | 8 (16.7%) | 4 (8.2%) | |
| Histology | 0.252 | ||
| Nonsquamous | 29 (60.4%) | 35 (71.4%) | |
| Squamous | 19 (39.6%) | 14 (28.6%) | |
| Stage | 0.967 | ||
| IIIB–IIIC | 7 (14.6%) | 7 (14.3%) | |
| IV | 41 (85.4%) | 42 (85.7%) | |
| Driven gene | 0.676 | ||
| EGFR mutation | 10 (20.8%) | 14 (28.6%) | |
| Other alterationsa | 10 (20.8%) | 9 (18.4%) | |
| Wild type | 28 (58.3%) | 26 (53.1%) | |
| ICIs line | 0.036 | ||
| 1 | 24 (50%) | 14 (28.6%) | |
| 2 | 12 (25%) | 24 (49%) | |
| ≥3 | 12 (25%) | 11 (22.4%) | |
| Treatment | 0.473 | ||
| Mono | 21 (43.8%) | 25 (51%) | |
| Combination | 27 (56.3%) | 24 (49%) | |
| ICI agent | 0.837 | ||
| Nivolumab | 15 (31.3%) | 15 (30.6%) | |
| Pembrolizumab | 23 (47.9%) | 27 (55.1%) | |
| Anti-PD-L1sb | 4 (8.3%) | 3 (6.1%) | |
| Anti-PD-1sc | 6 (12.5%) | 4 (8.2%) | |
| TAAb | 0.951 | ||
| Positived | 35 (72.9%) | 36 (73.5%) | |
| Negative | 13 (27.1%) | 13 (26.5%) |
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TAAb, tumor-associated autoantibody.
- a Other alterations include ALK (Anaplastic lymphoma kinase) fusion, RET (Ret proto-oncogene) fusion, and KRAS (Kirsten rat sarcoma viral oncogene homologue) G12C missense mutation.
- b Anti-PDL1s include atezolizumab, durvalumab, and sugemalimab.
- c Anti-PD-1s include sintilimab and tislelizumab.
- d The presence of any of the 35 tumor-associated antibodies in plasma was considered positive.
Identification of the 7-TAAb panel with predictive value for progression in the discovery cohort
To identify the TAAb panel with the best predictive value for ICI therapeutic efficacy, we performed the following procedures in the discovery cohort (Figure 1a). First, we screened the serum level of the 35 TAAbs of 48 patients. The results indicated that the number of positive cases of each TAAb ranged from 0 to 6, and TAAbs with no positive case were excluded (Figure 1b and Supporting Information Table S3). Second, we evaluated the median progression-free survival (mPFS) corresponding to the positive cases of each TAAb, and the TAAbs with mPFS less than 4 months were excluded (Figure 1C). Third, different combinations of TAAbs were designed with the remaining TAAbs, and the statistical correlation between their positivity with PFS was tested through Kaplan–Meier survival analysis (Supporting Information Table S4). Finally, a 7-TAAb panel composed of p53, CAGE, MAGEA4, GAGE7, UTP14A, IMP2, and PSMC1 was selected. Seventeen patients were positive, and the positivity of the panel showed significant association with better PFS (mPFS 9.9 vs. 4.3 months, p = 0.043, hazard ratio [HR] = 0.48, 95% confidence interval [CI] 0.25–0.93) (Figure 1D).
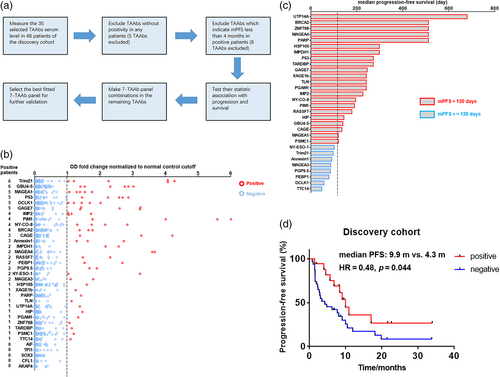
Evaluation of the performance of the 7-TAAb panel in the validation cohort
To examine the predictive value of the 7-TAAb panel in patients with different clinical backgrounds, we measured the serum level of the seven TAAbs in 49 patients of the validation cohort (Figure 2a and Supporting Information Table S5). Nineteen patients tested positive in the validation cohort, with a positive rate of 38.8%, similar to the discovery cohort (Figure 2b). Importantly, the positivity of this 7-TAAb panel was also significantly associated with longer PFS (mPFS 11.1 vs. 4.8 months, p = 0.015, HR = 0.40, 95% CI 0.21–0.83) (Figure 2C). Cox regression analysis showed that the positivity of this 7-TAAb panel was an independent predictor of PFS (p = 0.028) (Supporting Information Table S6), and patients with positive results had a 0.417-fold increased risk of disease progression after ICI-based treatment compared with patients with negative results. In the overall population including both discovery and validation cohorts, panel-positive patients had better prognosis than panel-negative patients, reflected by longer PFS (mPFS 10.9 vs. 4.3 months, p = 0.001, HR = 0.43, 95% CI 0.27–0.69) (Figure 2D) and higher objective response rate (ORR 36% vs. 23%) (Figure 2e).
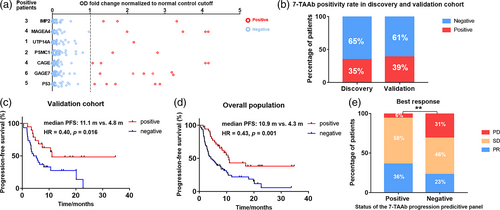
Evaluation of the predictive value of the 7-TAAb panel in different subgroups of the overall population
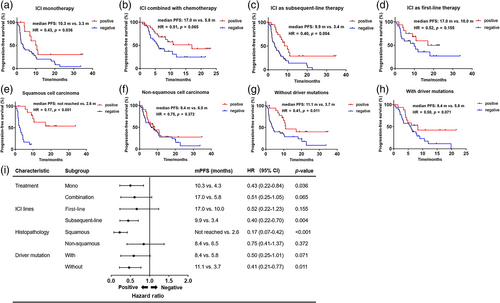
Besides the predictive value in general NSCLC patients, we were also interested in the performance of the 7-TAAb panel in different subgroups of patients classified by their distinct clinical characteristics. In the subgroup of patients treated with ICI monotherapy, longer PFS was observed in panel-positive patients (mPFS 10.3 vs. 4.3 months, p = 0.036, HR = 0.43, 95% CI 0.22–0.84) (Figure 3a). Furthermore, the 7-TAAb panel was also a good predictive biomarker for patients treated with ICI combined with chemotherapy (mPFS 17.0 vs. 5.8 months, p = 0.065, HR = 0.51, 95% CI 0.25–1.05) (Figure 3b). In addition, panel-positive patients who received subsequent-line ICI treatment had significantly longer PFS than panel-negative patients (mPFS 9.9 vs. 3.4 months, p = 0.004, HR = 0.40, 95% CI 0.22–0.70) (Figure 3C). A similar trend was observed in patients with first-line ICI treatment (mPFS 17.0 vs. 10.0 months, p = 0.115, HR = 0.52, 95% CI 0.22–0.70) (Figure 3D). Regarding histopathology, subgroup analysis revealed that the 7-TAAb panel worked better in patients diagnosed with squamous cell carcinoma (mPFS not reached vs. 2.6 months, p < 0.001, HR = 0.17, 95% CI 0.07–0.42) (Figure 3e), while no statistically significant difference was seen in the nonsquamous cell carcinoma subgroup (mPFS 8.4 vs. 6.5 months, p = 0.372, HR = 0.75, 95% CI 0.41–1.37) (Figure 3f). Moreover, for patients without driver mutations, the positivity of the 7-TAAb panel was significantly associated with longer PFS (mPFS 11.1 vs. 3.7 months, p = 0.011, HR = 0.41, 95% CI 0.21–0.77) (Figure 3g) and this association was observed in the subgroup with driver mutations as well (mPFS 8.4 vs. 5.8 months, p = 0.071, HR = 0.50, 95% CI 0.25–1.01) (Figure 3h). Overall, the positivity of the 7-TAAb panel was consistently associated with longer PFS in each intra-subgroup comparison (Figure 3I).
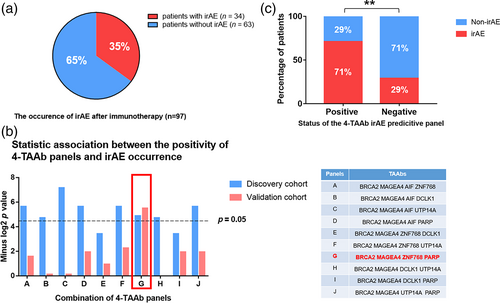
Identification of a 4-TAAb panel to predict the occurrence of irAEs
For all patients enrolled into the current study and followed up for more than 6 months (n = 97), the irAE occurrence rate was 35% (Figure 4a). We found that in the discovery cohort, only a single TAAb for BRCA2 had a statistically significant association with the occurrence of irAE after immunotherapy (Supporting Information Table S7), thus a panel of TAAbs with predictive value should be developed instead of a single TAAb to predict the irAE incidence. The development procedures for the TAAb panel were as follows: (1) TAAbs for BRCA2 and MAGEA4 with the smallest p value were selected for the panel; (2) two of the other five autoantibodies with relatively small p values were selected and combined with BRCA2 and MAGEA4 to form different 4-TAAb panels. The statistical associations between the positivity of each panel and the occurrence of irAE were tested by chi-square test, and only one panel (BRCA2, MAGEA4, ZNF768, and PARP) exhibited significant association in both the discovery and validation cohorts (Figure 4b and Supporting Information Table S8). Binary logistic regression analysis demonstrated that the panel was an independent predictor of irAEs after immunotherapy, and the risk of irAEs after immunotherapy in patients with positive results was 6.146 times higher than in patients with negative results (71.43% vs. 28.91%, p = 0.0046, 95% CI 1.755–21.52) (Figure 4C).
DISCUSSION
There is an urgent need to develop effective and convenient biomarkers to predict the therapeutic response to immunotherapy. In this study, we identified a panel of seven TAAbs in a discovery cohort and subsequently confirmed its predictive value in a validation cohort. The positivity of this 7-TAAb panel was an independent predictor of PFS in NSCLC patients treated with ICIs. In the subgroups of ICIs monotherapy, subsequent-line ICIs treatment, squamous carcinoma, and negative-driven gene mutation the 7-TAAb panel also worked well. Furthermore, in the present study we also identified another 4-TAAb (BRCA2 ZNF768 PARP MAGEA4) panel which was associated with irAE occurrence. The risk of irAE in patients with positive results from the 4-TAAb panel was higher than that in patients with negative results.
The seven TAAbs (p53, CAGE, MAGEA4, GAGE7, UTP14A, IMP2, and PSMC1) which we identified as therapeutic biomarkers have certain characteristics in common: overexpression or abnormal expression in tumor tissues, strong proto-oncogene function, and immunogenicity. The positivity of these TAAbs can be indicative of robust immune response at the tumor site after immunotherapy because it may reflect not only the high immunogenicity of corresponding TAAs, but also active humoral immune response and T cell-mediated immunity in the tumor microenvironment. For instance, higher levels of both p53 expression and anti-p53 antibodies were reported to be associated with longer survival,28, 29 and one possible explanation is that early accumulation of p53 mutations can activate immune responses to eliminate tumor cells effectively.30 CAGE and GAGE7 are cancer-testis antigens (CTAs), which are normally expressed in the reproductive system, but abnormally expressed in a variety of solid tumors.31 These CTAs are recognized as potential immunotherapeutic targets.32 Moreover, the 7-TAAb panel could also be compared between patients treated with ICIs and chemotherapy so we could test whether its predictive value is dependent on ICI treatment in a more rigorous way.
In addition, we characterized the other four TAAbs (BRCA2, ZNF768, PARP, and MAGEA4) as an independent predictor of irAE after immunotherapy, which are commonly featured by extensive expression in normal tissues. The positivity of these TAAbs may represent the enhanced immune reaction and damage to normal tissues after immunotherapy, thus the positive patients are more likely to develop irAEs. For example, the DNA transcriptional factor ZNF768 and the DNA damage repair protein BRCA2 are both widely expressed in lung, skin, skeletal muscle, and other organs,33, 34 and correspondingly in this study patients with positive ZNF768 TAAb developed interstitial pneumonia and patients with positive BRCA2 TAAb developed rash, bone pain, and severe anemia. Interestingly, although MAGEA4 is a tumor-derived antigen with oncogenic roles in many cancer tissues, including NSCLC,35 it is also related to the occurrence of irAEs, probably due to the remarkably high average serum titer of MAGEA4 TAAb compared to other TAAbs, which reflects not only the amount of aberrant products released from the tumor boosting the antitumor immunity, but also the abnormality of the overall state of the immune system causing damage to normal tissues.
It is worth noting that the results of our subgroup analysis suggested that the different clinical backgrounds of patients should also be taken into consideration when using the TAAb panel to predict clinical outcomes. We found that our 7-TAAb panel showed a consistent trend that the positivity was associated with longer PFS in all subgroups, but this feature was more pronounced in subgroups with certain characteristics. Notably, the intersubgroup difference could be due to normal deviation caused by relatively small sample size, but also may reflect the intrinsic difference between different subgroups of patients. For example, the predictive value of our panel was significantly better in the squamous cell carcinoma subgroup, with HR of 0.17, which is much lower than the 0.75 in the nonsquamous cell carcinoma group. This finding is consistent with previous studies which reported higher immune indicators such as PD-L1 expression, tumor-infiltrating cells, and ultimately better immune efficacy in squamous cell carcinoma compared with adenocarcinoma.36, 37 Furthermore, our subgroup analysis on driver mutation status also coincided with reported evidence that ICI-based treatments are more effective under conditions without driver mutations.38, 39 In general, the intersubgroup evaluation effectiveness difference of the TAAb panel can be attributed to patient-specific factors such as histological subtype, previous treatments, germline genetic variations, and other environmental factors,40, 41 which reminds us to comprehensively consider all factors including not only the immune indicator levels, but also other clinical characteristics, so that we can obtain more reliable results when predicting the efficacy of immunotherapy in clinical practice.
In conclusion, we have developed two TAAb panels to predict the therapeutic response and adverse events for advanced NSCLC patients after ICI-based treatment. Our data strongly support these TAAbs as promising immunotherapy biomarkers, and they should be further prospectively examined in a larger cohort to promote their ultimate application in clinical practice.
AUTHOR CONTRIBUTIONS
Conceptualization: Jing Zhao, Yang Wu, Yuan Yue, and Mengzhao Wang. Methodology: Jing Zhao, Yang Wu, and Yuan Yue. Data curation: Jing Zhao, Yang Wu, and Yuan Yue. Formal analysis: Jing Zhao, Yang Wu, and Yuan Yue. Visualization: Jing Zhao, Yang Wu, and Yuan Yue. Original draft and review: Jing Zhao, Yang Wu, Yuan Yue, Minjiang Chen, Yan Xu, Xiangning Liu, Xiaoyan Liu, Xiaoxing Gao, Hanping Wang, Xiaoyan Si, Wei Zhong, Xiaotong Zhang, Li Zhang, and Mengzhao Wang. The work reported in this paper has been performed by the authors, unless clearly specified otherwise in the text.
ACKNOWLEDGEMENTS
We would like to thank Hangzhou Cancer Probe Biotechnology Company for their technical support and contribution to this study. The authors would also like to thank the patients who contributed samples to the study as well as all the clinicians and staff for their efforts in collecting tissues and clinical information.
FUNDING INFORMATION
This study was supported by the CAPTRA-Lung Research Funds (No. CAPTRALung2021005), National High Level Hospital Clinical Research Funding (No, 2022-PUMCH-B-106) and the CAMS Innovation Fund for Medical Sciences (CIFMS 2018-I2M-1-003, to Jing Zhao).
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
The study was conducted in accordance with the Declaration of Helsinki. The study was approved by the ethics committee of the Peking Union Medical College Hospital (No. JS-1410) and informed consent was taken from all the patients.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are included within the article. Other raw data are available from the corresponding author upon reasonable request.




