Uniportal VATS lobectomy versus thoracotomy lobectomy for NSCLC larger than 5 cm: A propensity score-matched study
Abstract
Background
The performance of uniportal VATS lobectomy (uVATS) for non-small cell lung cancer (NSCLC) larger than 5 cm is uncertain due to a lack of evidence. Here, we present a retrospective, propensity-score matched cohort study to evaluate the safety and effectiveness of uVATS for patients with locally advanced NSCLC.
Methods
The data of patients with NSCLC larger than 5 cm diameter who underwent curative resection via uVATS or thoracotomy lobectomy between January 2015 and December 2020 was collected. Propensity-score matching was utilized to control the observable biases.
Results
Seventy-two patients underwent uVATS lobectomy, while 38 received thoracotomy lobectomy. No conversion to open surgery or perioperative death occurred. uVATS lobectomy achieved similar total lymph node dissection counts compared to thoracotomy and even yielded a higher amount of lymph node dissection in pTNM stage II patients. The long-term overall and recurrence-free survival rates were also similar between the two groups. Results from the propensity-score matching generated cohort agreed with those from the full cohort.
Conclusions
uVATS lobectomy is feasible and effective for curative lobectomy for NSCLC larger than 5 cm in diameter in selected patients. Further validations from well-designed prospective studies are required for uVATS lobectomy for patients with locally advanced NSCLC.
INTRODUCTION
Numerous advantages of video-assisted thoracic surgery (VATS) surgery, such as lower mortality, reduced postoperative pain, shorter hospital stay, and better quality of life for patients, have been recognized compared to traditional thoracotomy for non-small cell lung cancer (NSCLC).1, 2 The development of VATS and uniportal VATS (uVATS), first described by Rocco et al.,3 has gained a great deal of attention, and the indication for uniportal VATS surgery has rapidly expanded from minor procedures such as biopsy and wedge resection to complex operations such as lobectomy, segmentectomy, and even bronchoangioplasty.4
However, while the “territory” of uVATS continues to expand,5 one should adequately distinguish the exploratory nature so far of most of the accomplishments of uVAT. Furthermore, because the evidence for the use of uVATS is currently at a preliminary stage,6 the indications for uVATS remain far from clear. As the current consensus suggests, the indication for uVATS is now limited to patients with early stage NSCLC; that is, T1-2N0-1M0 disease, in the opinion of the majority of surgeons who practice uVATS.7 It is acknowledged that the compliance of tissue tends to decrease as the tumor enlarges, and a more extended lymphadenectomy would probably be required for tumor cell eradication and staging; the efficacy of uVATS for locally advanced NSCLC is yet to be confirmed. Here, we conducted a retrospective, propensity-score matched cohort study to compare the perioperative and long-term outcomes of uVATS and muscle-sparing thoracotomy lobectomy for locally advanced NSCLC, with the aim of delineating the proper indication for uVATS.
METHODS
Study population
The records of patients with pathological T3-4N0-2M0 NSCLC that underwent curative surgery at Peking University First Hospital between January 1, 2015, and December 31, 2020 were retrospectively collected and analyzed. The ethics committee of Peking University First Hospital reviewed and approved the study, and consent was acquired for each participant.
Eligibility criteria included: (1) Patients aged 18-years or older; (2) NSCLC pathology was confirmed; (3) curative lobectomy and lymphadenectomy received; and (4) no distant metastasis was indicated before the surgery. Exclusion criteria included: (1) Small cell histological types; (2) tumor invasion to adjacent lobe; and (3) radical resection not completed.
Staging and treatment
Preoperative examination included computed tomography (CT) of the chest and upper abdomen, ultrasonography of superficial lymph nodes, cranial magnetic resonance imaging (MRI), ultrasonic cardiogram, electrocardiogram, and pulmonary function test. Positron-emission tomography (PET)-CT was routinely recommended. Whether bronchoscopy was required was at the discretion of the surgeon. Staging was carried out according to the TNM staging system of AJCC eighth edition.
Following the surgical principle that the incision design and the sequence of dissection must conform to the anatomic configuration, we, under Jian Li's guidance, developed a set of surgical plans attuned to the anatomic composition of each lobe; that is, bronchus-artery–vein posteroanteriorly of the right upper lobe (RUL), artery-bronchus-vein posteroanteriorly of the right middle (RML) and the left upper lobes (LUL), and the artery-bronchus-vein cranial-caudally of the lower lobes, to accommodate our operative maneuvers. For uVATS surgery, an incision, typically 4–6 cm, was made in the anterior third intercostal space, for the right upper, lower, and left upper lobes, and the anterior fourth intercostal space, for the lower lobes, anterior intercostal space collateral ipsilateral to the lesion. The thoracic cavity was first explored to exclude pleural metastasis. The lesion-bearing lobe was maneuvered by a gauge-protected thoracoscopic clamp to reduce the compression of the tumor to prevent iatrogenic spread of malignant cells. A one-way method was applied to dissect the tumor-bearing lobe. The basic principle was to dissect the hilar structures away from the tumor. For a tumor residing in the right upper lobe, the division and dissection were started either from the bronchus then anteriorly, or from the vein then posteriorly, and for the left upper lobe and right middle lobe, from the pulmonary arteries then anteriorly or from the vein then posteriorly. For the right and left lower lobes, from the pulmonary arteries then caudally, or from the pulmonary vein then cranially. For the thoracotomy approach, a muscle-sparing technique was routinely applied. An 8–10 cm incision was created in the anterior fourth intercostal space. The anterior serratus muscle and the latissimus dorsi muscle were dissected and protected, and through the upper edge of the fifth rib, the thoracic cavity was entered in an intercostal nerve-preserving fashion. Once the thoracic cavity was explored and metastasis was ruled out, the lobectomy was conducted in a fissure-first manner. A one-directional method would be applied if the fissure was severely undeveloped. Other maneuvers followed the same principle of the uVATS lobectomy.
The relevant station 10, 11, and 12 lymph nodes were usually completely resected with the aid of a rubber tip sheathed suction apparatus, used to bluntly pull the lobar vessels or the lymph nodes to be resected. Because the tumor burden of patients in our study was already heavy enough, we routinely dissect the ipsilateral mediastinal lymph nodes. The contralateral hilar and the pretracheal lymph nodes could also be dissected by our surgical technique if needed. The dissected lymph nodes were retrieved using the cropped finger part of rubber gloves. The specimen, that is, the tumor-bearing lobe, was loaded in the retrieving plastic bag inside the thoracic cavity, then pulled out through the incision. For tumors that did not fit the intercostal space, we would then try in-bag dimension reduction to facilitate retrievement. If dimension reduction proved ineffective, we would lengthen the incision up to 6 mm. Rib-spreading was reserved as a last resort, which, fortunately, has not yet been used for specimen retrieval in our study.
A thoracic tube was routinely placed in the pleural cavity at the end of the operation. The patient would receive postoperative treatment, typically on the first to third postoperative day, in the intensive care unit if needed, or returned to our ward if not. Routine postoperative treatments consist of prophylactic antibiotic administration, intravenous patient-controlled anesthesia, and apophlegmatic treatments. The blood routine and biochemistry tests were conducted every other day. The thoracic tube would be removed if (1) the water-sealed level remained still when the patient coughed, (2) the volume of the drainage fluid was less than 200 ml 24 h prior to removal, and (3) the color of the drainage fluid was mellow yellow colored. The patient was discharged the day after thoracic tube removal if no discomfort was reported.
Follow-up and outcome
After discharge, the patient would receive follow-up every 3 months in the first 2 years, then every 6 months in the 3–5 years. Chest CT, ultrasonography of the abdomen and superficial lymph nodes, head MRI and serum tumor marker test were performed at each follow-up. The primary outcomes of our study were OS and RFS. The OS rate was calculated from the date of the surgery to the date of death of any cause, and the RFS rate was calculated from the date of the surgery to the date of disease recurrence. The event-free patient at the last available follow-up date was right-censored at this date in the survival analysis.
Propensity score matching
Propensity score matching (PSM) analysis, a method that could limit the bias caused by an existing dataset for nonrandom assignment analysis,8 was utilized to minimize the inherent bias in the retrospective study. Propensity scores were calculated using logistic regression based on the patient's preoperative characteristics, including age, sex, body mass index, smoking index, forced expiration volume, and diffusion capacity of the carbon monoxide. A 1:1 matched cohort was generated by matching patients who underwent the uVATS and the thoracotomy approach using a caliper width equal to 20% of the standard deviation of propensity scores without replacement. The post-matching balance was tested by student's t-test or Wilcoxon rank-sum test for continuous variable and the chi-square test or Fisher's exact test for categorical variables.
Statistical analysis
The OS and RFS of patients who underwent uVATS and the thoracotomy approach were calculated and compared via the Kaplan–Meier method in the overall study population and the PS-matched cohort. A variable would be included in multivariable Cox regression if univariable Cox regression suggested significance.
The normality of continuous variables was tested by the Shapiro-Wilk test. Student's t-test and Wilcoxon rank-sum test were applied to compare the normal and skewed distributed continuous variables between groups, respectively. The chi-square test or Fisher's exact test was performed for categorical variables. For all analyses, a p-value <0.05 in a two-tailed test was considered statistically significant. All analyses were performed with STATA/MP 15.1 software (StataCorp LLC), the R Project for Statistical Computing,9 and the R studio.
RESULTS
Patient characteristics
A total of 110 NSCLC patients who underwent either uVATS or thoracotomy approach lobectomy were identified, including 72 patients who underwent uVATS and 38 patients who underwent thoracotomy. The patients who received uVATS were younger, had a higher BMI, and fewer underwent preoperative bronchoscopy. There were more patients with squamous cell cancer in the thoracotomy group. PSM generated 23 pairs of patients, whose preoperative characteristics were well-balanced (Table 1).
| Full cohort (N = 110) | Matched cohort (N = 46) | |||||
|---|---|---|---|---|---|---|
| uVATS (N = 72) | Thoracotomy (N = 38) | p-value | uVATS (N = 23) | Thoracotomy (N = 23) | p-value | |
| Age | 62.5 (56–67) | 67 (60.75–71) | 0.009 | 62.91 (1.69) | 62.95 (1.75) | 0.986 |
| Body mass Index | 25.24 (0.44) | 23.25 (0.63) | 0.011 | 24.53 (0.81) | 24.61 (0.81) | 0.949 |
| Sex | 0.372 | 1.000 | ||||
| Male | 61 (85%) | 35 (92%) | 21 (91%) | 20 (87%) | ||
| Female | 11 (15%) | 3 (8%) | 2 (9%) | 3 (13%) | ||
| Smoking Index | 20 (0–40) | 52 (0–50) | 0.254 | 40 (0–45) | 20 (0–40) | 0.622 |
| Comorbidities | 0.782 | 0.765 | ||||
| No | 38 (53%) | 19 (50%) | 14 (61%) | 13 (57%) | ||
| Yes | 34 (47%) | 19 (50%) | 9 (39%) | 10 (43%) | ||
| ECOG | 0.175 | 1.000 | ||||
| 0 | 56 (78%) | 25 (66%) | 18 (78%) | 17 (74%) | ||
| 1 | 16 (22%) | 13 (34%) | 5 (22%) | 6 (26%) | ||
| PET-CT | 0.111 | 0.369 | ||||
| No | 42 (58%) | 28 (74%) | 12 (52%) | 15 (65%) | ||
| Yes | 30 (42%) | 10 (26%) | 11 (48%) | 8 (35%) | ||
| Bronchoscopy | 0.047 | 1.000 | ||||
| No | 70 (97%) | 35 (92%) | 22 (96%) | 21 (91%) | ||
| Yes | 2 (3%) | 3 (8%) | 1 (4%) | 2 (9%) | ||
| Neoadjuvant | 0.202 | 1.000 | ||||
| No | 69 (96%) | 38 (100%) | 22 (96%) | 23 (100%) | ||
| Yes | 3 (4%) | 0 (0%) | 1 (4%) | 0 (0%) | ||
| FEV1% | 92.85 (1.99) | 81.99 (2.71) | 0.002 | 88.15 (3.83) | 90.12 (2.62) | 0.674 |
| DLCO% | 88.2 (75.82–98.82) | 82 (68.22–96.90) | 0.148 | 86 (71.2–98.3) | 86.4 (75–99.3) | 0.637 |
- Note: Variables are presented as mean ± standard deviation, median (first quartile – third quartile), or n (%). FEV1%, the percent of actual forced expiratory volume in 1 s divided by predicted forced expiratory volume in 1 s. DLCO%, the percent of actual diffusing capacity of the lung for carbon monoxide divided by the predicted diffusing capacity of the lung for carbon monoxide.
- Abbreviations: ECOG, Eastern Cooperative Oncology Group; PET-CT, positron-emission tomography-computed tomography; PS, propensity score; uVATS, uniportal video-assisted thoracic surgery.
Perioperative outcomes
Perioperative outcomes of the whole and the PS-matched cohorts are presented in Table 2. The uVATS groups showed a significantly less thoracic drainage tube placement time compared to the thoracotomy groups (p = 0.013), which did not persist in the PS-matched cohort. The operative time and bleeding of uVATS groups were less compared to the thoracotomy group without statistical significance nonetheless (p = 0.496 and p = 0.110, respectively). The amount of lymph nodes dissected in N1, and N2 stations and the amount of N2 stations dissected were also comparable in both the whole and the PS-matched cohorts (Table 2). In the PS-matched cohort, the number of N1 lymph nodes dissected in the uVATS group was even greater than that in the thoracotomy groups (Figure 1).
| Full cohort (N = 110) | Matched cohort (N = 46) | |||||
|---|---|---|---|---|---|---|
| uVATS (N = 72) | Thoracotomy (N = 38) | p-value | uVATS (N = 23) | Thoracotomy (N = 23) | p-value | |
| Lobe | 0.794 | 0.913 | ||||
| RUL | 24 (33%) | 11 (29%) | 8 (35%) | 8 (35%) | ||
| RML | 0 (0%) | 1 (3%) | 0 (0%) | 0 (0%) | ||
| RLL | 16 (22%) | 9 (24%) | 4 (17%) | 5 (22%) | ||
| LUL | 16 (22%) | 8 (21%) | 6 (26%) | 4 (17%) | ||
| LLL | 16 (22%) | 9 (24%) | 5 (22%) | 6 (26%) | ||
| First structure dissected | 0.746 | 1.000 | ||||
| Pulmonary vein | 15 (21%) | 5 (13%) | 3 (13%) | 3 (13%) | ||
| Bronchus | 2 (3%) | 1 (3%) | 1 (4%) | 1 (4%) | ||
| Pulmonary Artery | 55 (76%) | 32 (84%) | 19 (83%) | 19 (83%) | ||
| Suture or stapled | 0.086 | 0.017 | ||||
| Suture | 37 (51%) | 26 (68%) | 9 (39%) | 17 (74%) | ||
| Stapled | 35 (49%) | 12 (32%) | 14 (61%) | 6 (26%) | ||
| Vessel plastry | 0.532 | 0.381 | ||||
| 0 | 62 (86%) | 31 (82%) | 21 (91%) | 19 (83%) | ||
| 1 | 10 (14%) | 7 (18%) | 2 (9%) | 4 (17%) | ||
| Operative bleeding | 100 (50–287.5) | 200 (100–262.5) | 0.246 | 100 (50–150) | 200 (100–200) | 0.110 |
| Operative time | 229 (195.75–294.5) | 264 (209.5–328.75) | 0.075 | 248 (188–284) | 262 (203–307) | 0.496 |
| Operative RBC | 0 (0–0) | 0 (0–0) | 0.498 | 0 (0–0) | 0 (0–0) | 0.317 |
| Operative plasma | 0 (0–0) | 0 (0–0) | 0.644 | 0 (0–0) | 0 (0–0) | 1.000 |
| N1 dissection | 4 (0–10) | 1 (0–8.25) | 0.207 | 4 (0–8) | 1 (0–8) | 0.493 |
| N2 dissection | 12 (8–16.75) | 10.5 (8–16.25) | 0.763 | 11 (6–16) | 11 (8–15) | 0.488 |
| N dissection | 20.944 (1.061) | 20.131 (1.457) | 0.653 | 18.652 (1.428) | 20.739 (1.73) | 0.357 |
| N2 station dissection | 3.5 (3–4) | 4 (3–4) | 0.371 | 3.521 (0.187) | 3.869 (0.181) | 0.189 |
| Thoracic drainage | 5 (3–6.75) | 6 (4–9) | 0.013 | 4 (3–6) | 6 (4–8) | 0.116 |
| Complication (CD) | 0.526 | 0.459 | ||||
| 0 | 61 (85%) | 31 (82%) | 17 (74%) | 19 (83%) | ||
| 2 | 11 (15%) | 6 (16%) | 6 (26%) | 3 (13%) | ||
| 3a | 0 (0%) | 1 (3%) | 0 (0%) | 1 (4%) | ||
- Note: Variables are presented as mean ± standard deviation, median (first quartile – third quartile), or n (%). Complications are presented by the Clavien-Dindo classification of surgical complications.
- Abbreviations: CD, Clavien-Dindo classification; LLL, left lower lobe; LUL, left upper lobe; PS, propensity score; RBC, red blood cell count; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; uVATS, uniportal video-assisted thoracic surgery.

Survival outcomes
The median follow-up period was 35 months (range 6–89 months), the 5-year OS was 55.64%, and the 5-year RFS was 53.72%. In the whole cohort, the 5-year OS rate of the uVATS group was 54.39%, and the right approach group was 58.29% (p = 0.775) (Figure 2a). The 5-year RFS rate of the left and right approach groups were 51.62% and 58.05% (p = 0.352), respectively (Figure 2b). After PS matching, the 5-year OS rates of the left and right approach groups were 39.09% and 50.78% (p = 0.501, respectively (Figure 2c), the 5-year RFS rates were 35.51% and 50.47% (p = 0.316), respectively (Figure 2d).

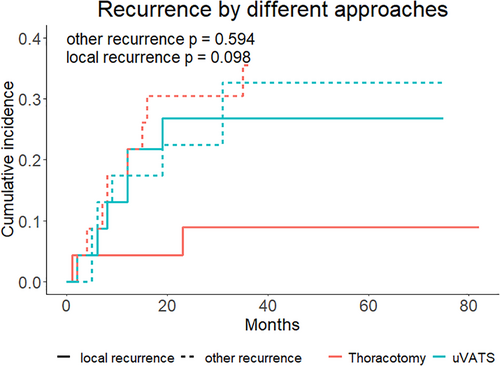
We investigated the pattern of recurrence in the groups using competing risk analyses. No statistical difference in the local recurrence rate was found in the analysis between the uVATS and thoracotomy groups (Figure 3). In the multivariate Cox regression analysis, a higher diffusion capacity for carbon monoxide, more intraoperative bleeding, and the presence of pathological nerve invasion were independently associated with worse OS and RFS (Table 3).
| Variables | Hazard ratio | p-value | 95% confidence interval | |
|---|---|---|---|---|
| Overall survival | ||||
| DLCO% | 1.012 | 0.010 | 1.002 | 1.021 |
| Intraoperative bleeding | 1.001 | 0.035 | 1.000 | 1.002 |
| Nerve invasion | 2.596 | 0.040 | 1.045 | 6.449 |
| Recurrence free survival | ||||
| DLCO% | 1.011 | 0.017 | 1.002 | 1.021 |
| Intraoperative bleeding | 1.001 | 0.016 | 1.000 | 1.002 |
| Nerve invasion | 2.667 | 0.019 | 1.178 | 6.037 |
- Note: DLCO%, the percent of actual diffusing capacity of the lung for carbon monoxide divided by the predicted diffusing capacity of the lung for carbon monoxide.
DISCUSSION
The safety and effectiveness of VATS in treating early stage NSCLC has been previously reported to a relatively satisfactory degree since the advent of VATS surgery.1, 10-12 The treatment of NSCLC larger than 5 cm is, on the other hand, a different topic, as the hindrance of operative maneuvers from the large tumor and the higher requirement of the lymphadenectomy calls for more adequate and precise resection. Albeit the effectiveness and safety of VATS lobectomy has been previously investigated,8, 13, 14 the question of whether uVATS is feasible for NSCLC larger than 5 cm is far from clear. As the first study to examine the safety and effectiveness of uVATS in this clinical setting, we determined that: (1) uVATS could be used to conduct lobectomy for NSCLC larger than 5 cm in a safe and efficient manner, and the operative time, intraoperative bleeding volume, and postoperative complication rate were comparable to thoracotomy lobectomy. (2) The uVATS could reach satisfactory oncological effects compared to thoracotomy lobectomy, the amount of lymph nodes resected and the long-term survival rates were comparable between the two groups.
The volume of the available part of the thoracic cavity decreases when the tumor grows larger, thus the space for operative maneuvers lessens. The physical changes brought by a large tumor also contribute to the difficulties of field exposure and tissue manipulation, which would be more prominent under the uVATS setting. For uVATS under this setting, there are two principles worth mentioning. First, to avoid intraoperative hemorrhage and iatrogenic tumor spreading, the sequence of exposure and dissection of hilar structures should conform to the spatial configuration of the hilum of the tumor-bearing lobe, we recommend exposing and dissecting the hilar structures in a unidirectional fashion, for example, a vein–artery-bronchus sequence for the right upper and middle lobes and the vein-bronchus-artery sequence for the left upper lobe. Second, the anterior third intercostal space, which shortens the distance between the port and the lung hilum the most, could be a proper choice for uVATS surgery that bears a greater risk of major intraoperative hemorrhage or other complications that require complex manipulations of hilar structures.
Following those principles, the uVATS lobectomy for NSCLC larger than 5 cm could be conducted with enough safety and efficiency. As previously published studies have shown, the operative duration of VATS surgery for NSCLC larger than 5 cm was comparable (Nakano et al., 169.4 ± 48.6 vs. 186.0 ± 58.9 min, p = 0.21; Batihan et al., 228.75 ± 91.88 vs. 260.17 ± 102.78 min, p = 0.784), if not significantly shorter (Bu et al., 186.5 ± 62.8 vs. 256.7 ± 67.5 min, p < 0.001), than that of the thoracotomy surgery. Our results also suggested that the operative duration of uVATS lobectomy tended to be shorter than that of the thoracotomy lobectomy in the PS-matched cohort (248 [188–284] vs. 262 [203–307] min, p = 0.496). The absence of significance probably resulted from our position in the learning curve of the uVATS, as the safety, other than the speed, of an operation is of the utmost importance.
Our study also showed trends of less operative bleeding in the uVATS group compared to the thoracotomy group in both the whole (100 [50–287.5] vs. 200 [100–262.5] ml, p = 0.246) and the PS-matched (100 [50–150] vs. 200 [100–200] ml, p = 0.110) cohorts, which is not in accordance with the results from Bu et al. and Nakano et al. The trend, other than a significant difference, of less intraoperative bleeding between the groups possibly stems from a narrow effect size and a limited sample size as the intraoperative blood loss was already controlled at an insignificant level. In addition, of the thoracic drainage time, the uVATS groups were even shorter in the whole cohort compared to the thoracotomy group, suggesting the mini-invasiveness potential of the uVATS.
The oncological effects of radical tumor resection are, of course, a crucial part of operative technique evaluation. One inevitable topic of radical resection for NSCLC is lymphadenectomy. Needless to say, a complete lymphadenectomy, which naturally would be warranted for radical resection for NSCLC with greater size, calls for more careful traction, manipulation, and dissection of the perinodal tissue. The amount of lymph nodes resected, whether in stage II or stage III, was comparable between the groups in our study (Table 2), suggesting the competency of the uVATS for lymphadenectomy.15 Taking a look back into the progression of lymphadenectomy for NSCLC, one could find out that the controversies apropos of the survival benefit of lymphadenectomy for NSCLC shared almost the same longevity with the concept of lymphadenectomy itself.9 The one goal of a thorough lymphadenectomy is a more precise staging, hitherto recognizing patients who could benefit from adjuvant therapy.16, 17 However, the value of this kind of recognition is limited in our study, since the study population of our research requires some degree of adjuvant therapy uniformly. However, we believe that the meticulously conducted lymphadenectomy could bring a certain set of patients, with resectable pN1 and pN2 disease and no distant micrometastasis, survival benefits as suggested by previous studies.18-20
Finally, at the very core of every radical resection for malignancy lies the evaluation of the long-term results. As technical difficulties of surgical resection for large NSCLC were documented in previous studies8, 13, 14 as well as in our discussion, satisfactory results including similar OS and RFS rates in both the whole and the PS-matched cohort were obtained. However, a marginal, nonsignificant difference in the local recurrence rate was observed in the competing-risk analysis for the recurrent pattern. Currently, whether this difference is derived from both approaches or the random error is unfortunately unclear. We hope that this question will be answered in future studies.
Our study has several limitations. First, the single-center, retrospective nature of our study limited the level of evidence of our results from the beginning, while the PS-matching could control some, but not all, of the bias. Second, the sample size of our study was limited. Third, pre- and postoperative therapy records were not detailed enough to facilitate a more specific analysis. Hopefully, those problems will be addressed in a future, multicenter, large-scale prospective randomized controlled trial.
In conclusion, our study discovered that compared to the thoracotomy lobectomy, uVATS lobectomy is safe and efficient for NSCLC larger than 5 cm. According to the results, we encourage the application of uVATS lobectomy for NSCLC larger than 5 cm when the resectability has been confirmed by preoperative study and sufficient experience of VATS surgery has been obtained. However, conversion to multiportal VATS or open surgery should be considered whenever the risk of intraoperative bleeding or incomplete resection emerges.
AUTHOR CONTRIBUTIONS
JL: conceived and designed the study. JWL, XNZ: data collection and processing, analysis of the data. JWL: wrote the manuscript. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGMENT
All authors acknowledge that no funding or support was received.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.




