Feasibility and safety of secondary video-assisted thoracoscopic surgery for ipsilateral lung cancer after prior pulmonary resection
Funding information: Sichuan Provincial Department of Science and Technology Program of Sichuan Province, Grant/Award Number: 2021YFS0024
Abstract
Background
Video-assisted thoracoscopic surgery (VATS) is the preferred treatment for resectable non-small cell lung cancer. The increased survival of patients after a first operation has caused increases in the incidence of locoregional recurrence or second primary lung cancer and a concomitant increase in the number of patients who require secondary surgery. Ipsilateral secondary operation is also commonly practiced, albeit with enhanced difficulty. Therefore, it is necessary to evaluate the feasibility and safety of VATS for ipsilateral lung cancer after pulmonary resection.
Methods
Patients who underwent ipsilateral secondary VATS in the West China Hospital, Sichuan University from 2012 to 2021 were assessed retrospectively. All included patients had a pulmonary resection. Clinical characteristics, perioperative outcomes, and survival data were collected, with an emphasis on conversion to thoracotomy, postoperative complications, 30-day mortality, and survival. Logistic regression analysis was used to identify predictors of postoperative complications.
Results
Seventy patients were enrolled, of which 10 (14.3%) had converted thoracotomy, 17 (24.3%) had postoperative complications, and two (2.9%) had grade III complications. No patient died within 30 days after surgery. High Charlson comorbidity index (CCI) and severe pleural adhesion were independent predictors for complications. The median follow-up was 50 months (range: 3–120), and the 5-year overall survival was 78.2%.
Conclusion
Secondary VATS for ipsilateral lung cancer for patients who had pulmonary resection was feasible and safe. Strict preoperative evaluation and careful management of pleural adhesion are crucial for the success of the surgery.
INTRODUCTION
Minimally invasive surgery for lung cancer has gradually replaced traditional thoracotomy since 1992 when Lewis first reported the use of video-assisted thoracoscopic surgery (VATS) to treat lung cancer.1 Compared with traditional open surgery, VATS is less invasive and is associated with faster postoperative recovery. The advancement of modern medical imaging and the standardization of patient follow-up have promoted increased incidence of second primary lung cancer or locoregional recurrence after lung cancer surgery. Lung cancer patients have a 2%–10% chance of recurrence or developing a second primary lung cancer each year after a first pulmonary resection.2 Although there are many reports of satisfactory safety and survival of VATS for resectable lung cancer, these studies focused mainly on the first surgery. For surgeons, ipsilateral thoracoscopic reoperation is challenging due to severe pleural adhesions and altered hilar anatomy that result from the earlier surgery. Therefore, it is important to establish the feasibility and safety of VATS for ipsilateral lung cancer after pulmonary resection. In this study, we retrospectively analyzed patients who underwent secondary VATS for ipsilateral lung cancer to investigate the outcomes for patients who had prior pulmonary resection.
METHODS
The Institutional Review Board of West China Hospital approved this study. The data were drawn from the prospective Western China Lung Cancer Database (WCLCD) of the Department of Thoracic Surgery, West China Hospital, Sichuan University. The database is maintained by a professional team. Each patient signed informed consent at the time of inclusion in the WCLCD database. We consecutively reviewed the data of lung cancer patients who were included in the WCLCD during 2012–2021.
Patients
Inclusion criteria were: (1) Patients with a prior pulmonary resection who needed reoperation for ipsilateral locoregional recurrence or second lung cancer, (2) the second surgery was VATS, and (3) at least one lesion in the secondary surgery was pathologically confirmed as lung cancer. Patients were excluded if they had distant metastases or histologically confirmed pulmonary metastases from other cancers before the secondary surgery, or if they had a secondary open surgery.
Treatment
Preoperative workup
Preoperative workup included detailed history-taking and physical examination, chest computed tomography, and head and upper abdomen tomography or magnetic resonance imaging. Fiberoptic bronchoscopy was performed for patients with suspicious airway invasion. Some patients also underwent whole body bone scintigraphy or positron emission tomography-CT to exclude tumors of other origins. Patients aged ≥60 years and patients who had comorbid cardiovascular system disease also underwent echocardiography and coronary angiography. All patients had pulmonary function tests before the secondary surgery.
Operation
Each patient was intubated with a double-lumen tube and placed in the healthy-side lying position after general anesthesia. The VATS procedure is described in our previous reports.3 It is a completely endoscopic technique performed through one or three ports in the intercostal space, based entirely on the image. At our center, VATS lobectomy is performed mainly using the single-direction method as previously described.3 The method is convenient and extensible for pulmonary resection, which begins at the lung hila and is sequentially performed along a single direction from the surface to the inside. The drainage tube was removed after postoperative review of the chest x-ray to determine that there was no air leakage. If there were no other complications, the patient was discharged 1–2 days after all drainage tubes were removed. All resected specimens were sent for pathological analysis, and the results were used to determine postoperative management. The postoperative patients were followed on an outpatient basis every 3–6 months for the first 2 years, every 6 months for the next 3 years, and annually thereafter. Patients who were unable to visit the outpatient clinic were followed regularly by telephone.
Outcome assessment
Surgical feasibility and safety included the following details: (1) Conversion to thoracotomy, (2) postoperative complications graded according to Clavien–Dindo's method4 and (3) thirty-day postoperative mortality.
Statistical analysis
Categorical variables are described as numbers and percent, and continuous variables as medians and ranges. The continuous variables were converted into categorical variables with clinically significant boundary values for univariate analysis, and the variables with p < 0.1 in univariate analysis were included in logistic multivariate analysis to evaluate the influencing factors of postoperative complications. Kaplan–Meier curves were used for survival analysis. Overall survival was calculated from the second surgery until death or last follow-up. The last follow-up was June 2022. The tumor, node, and metastasis (TNM) staging was performed according to the International Association for the Study of Lung Cancer eighth edition staging criteria. The comorbidity score was classified using the Charlson comorbidity index (CCI).5 Pleural adhesions were defined as mild (less than 30%), moderate (30%–70%), and severe (more than 70%). Statistical analysis was performed using SPSS for Windows version 25.0 (SPSS, Inc.).
RESULTS
Patient characteristics
Seventy patients who had secondary ipsilateral VATS lung resection after a previous pulmonary surgery were included in this study. Table 1 shows patient characteristics. There were 38 (54.3%) men and 26 (37.1%) of the patients were smokers. The median age was 59.5 years (range: 32–77). Seven (10.0%) patients received chemotherapy after the first surgery. The median interval between the two operations was 23 months (range: 1.1–231). Thirty-three patients (47.2%) underwent lobectomy and 37 (52.8%) underwent sublobectomy for secondary surgery. Postoperative pathology showed that adenocarcinoma (n = 56) was the predominant histology, followed by squamous carcinoma (n = 6), small cell carcinoma (n = 1) and squamous plus adenocarcinoma (n = 1). There were 55 patients (78.6%) with stage I lung cancer, five (7.1%) with stage II, nine (12.9%) with stage III, and one (1.4%) with stage IVa. Most patients (97.1%) had pleural adhesions, including 29 with severe adhesions (Table 2). The median length of time for the secondary operation was 120 min (range: 30–472), and the median blood loss was 50 ml (range: 3–600). Ten patients had converted thoracotomy, all of which were due to difficulties in surgical operation caused by severe adhesion (Table 2).
| Variable | n = 70 |
|---|---|
| Age at second operation | 59.5 (32–77) |
| Sex | |
| Male | 38 (54.3%) |
| Female | 32 (45.7%) |
| Smoking status | |
| Never | 44 (62.9%) |
| Former/current | 26 (37.1%) |
| BMI (kg/m2) | 23.0 (18.3–30.1) |
| Pre-FEV1% | 95.8 (67.6–157.8) |
| CCI | 2 (0–4) |
| First operation | |
| Histology | |
| ADC | 51 (72.8%) |
| Other types of malignancies | 13 (18.6%) |
| Benign lesions | 6 (8.6%) |
| Procedure | |
| Lobectomy | 42 (60.0%) |
| Sublobectomy | 28 (40.0%) |
| Surgical approach in first operation | |
| VATS | 66 (94.3%) |
| Thoracotomy | 4 (5.7%) |
| Second operation | |
| Operation interval (months) | 23 (1.1–231) |
| Laterality | |
| Right | 46 (65.7%) |
| Left | 24 (34.3%) |
| Maximum tumor size (cm) | 1.5 (0.5–6.3) |
| Procedure | |
| Lobectomy | 33 (47.2%) |
| Sublobectomy | 37 (52.8%) |
| Lymph node dissection | |
| YES | 45 (64.3%) |
| NO | 25 (35.7%) |
| Histological type | |
| ADC | 56 (80%) |
| Other types of malignancies | 14 (20%) |
| Pathological TNM stagea | |
| I | 55 (78.6%) |
| II | 5 (7.1%) |
| III | 9 (12.9%) |
| IVa | 1 (1.4%) |
| Neoadjuvant chemotherapy | |
| YES | 7 (10%) |
| NO | 63 (90%) |
| Adjuvant chemotherapy | |
| YES | 24 (34.3%) |
| NO | 46 (65.7%) |
- Abbreviations: ADC, adenocarcinoma; BMI, body mass index; CCI, Charlson comorbidity index; Pre-FEV1%, percentage of forced expiratory volume in the first second of expiration for predicted values; VATS, video-assisted thoracoscopic surgery.
- a The tumor, node, and metastasis (TNM) stage was performed according to the International Association for the Study of Lung Cancer eighth edition staging criteria.
| Variable | n = 70 |
|---|---|
| Operation time (min) | 120 (30–472) |
| Blood loss | 50 (3–600) |
| Conversion to thoracotomy | 10 (14.3%) |
| Pleura adhesion | |
| None | 2 (2.9%) |
| Mild | 11 (15.7%) |
| Moderate | 28 (40.0%) |
| Severe | 29 (41.4%) |
| Postoperative hospital stay (days) | 6 (2–16) |
| Air leakage (lasting ≥7 days) | 9 (12.9%) |
| Pneumonia | 5 (7.1%) |
| Reinsertion of chest tube | 2 (2.9%) |
| Incision infection | 1 (1.4%) |
| Clavien-Dindo classificationa | |
| I | 10 (14.3%) |
| II | 5 (7.1%) |
| IIIa | 2 (2.9%) |
| In-surgery blood transfusion | 0 (0%) |
| 30-day mortality | 0 (0%) |
- a According to the Clavien–Dindo classification.
Postoperative complications
Seventeen patients (24.3%) developed postoperative complications. The main cause was air leakage (12.9%), followed by pneumonia (7.1%). Two patients (2.9%) with grade III complications had drainage tubes reinserted because of pneumothorax. The median postoperative hospital stay was 6 days (range: 2–16), and there was no postoperative 30-day death. Univariate analysis showed that smoking, surgical time ≥120 min, blood loss ≥100 ml, conversion to thoracotomy, high CCI, and severe pleural adhesions were significantly associated with postoperative complications. Variables with p < 0.1 were included in a logistic regression model for multivariate analysis. High CCI (OR = 4.167, 95% Cl: 1.071–16.211, p = 0.040) and severe pleural adhesions (OR = 13.000, 95% Cl: 3.243–52.112, p = 0.020) were independent predictors for postoperative complications. Other perioperative factors that did not affect the occurrence of complications were age, sex, BMI, laterality, solid component of the tumor, p-stage, histological types, maximum tumor diameter, time interval between the two operations, pre-FEV1, lymph node dissection, and neoadjuvant chemotherapy (Table 3).
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI) | p-v alue | OR (95% CI) | p-v alue | |
| Age (≥60) | 1.600 (0.529–4.837) | 0.405 | ||
| Sex (male) | 0.401 (0.124–1.298) | 0.127 | ||
| Smoking status (former/current) | 3.304 (1.067–10.226) | 0.038 | 2.675 (0.642–11.150) | 0.177 |
| BMI (≥24 kg/m2) | 1.728 (0.570–5.240) | 0.334 | ||
| Pre-FEV1% (<80%) | 2.222 (0.337–14.562) | 0.405 | ||
| CCI (≥2) | 4.167 (1.071–16.211) | 0.040 | 6.062 (1.120–32.804) | 0.036 |
| Interval time (≥6 months) | 1.726 (0.571–5.222) | 0.334 | ||
| Maximum tumor size (≥2 cm) | 1.773 (0.570–5.552) | 0.323 | ||
| Procedure first (lobectomy) | 1.376 (0.455–4.157) | 0.572 | ||
| Procedure second (lobectomy) | 1.004 (0.336–3.001) | 0.994 | ||
| Laterality (right) | 0.486 (0.159–0.489) | 0.207 | ||
| Operation time (≥120 min) | 4.494 (1.155–17.479) | 0.030 | 1.699 (0.245–11.776) | 0.592 |
| Blood loss (≥100 ml) | 4.396 (1.391–13.894) | 0.012 | 0.632 (0.093–4.278) | 0.638 |
| Conversion to thoracotomy (Yes) | 6.682 (1.608–27.759) | 0.009 | 1.899 (0.302–11.925) | 0.494 |
| Pleural adhesion (severe) | 13.000 (3.243–52.112) | <0.001 | 9.079 (1.426–57.823) | 0.020 |
| Neoadjuvant chemotherapy (Yes) | 1.280 (0.225–7.287) | 0.781 | ||
| Lymph node dissection (Yes) | 1.025 (0.327–3.210) | 0.967 | ||
| p-stage of the second lung cancer (II–IV)a | 1.792 (0.513–6.252) | 0.360 | ||
| Histology type (ADC) | 0.491 (0.138–0.741) | 0.271 | ||
- Abbreviations: ADC, adenocarcinoma; BMI, body mass index; Cl, confidence interval; CCI, Charlson comorbidity index; OR, odds ratio; Pre-FEV1%, percentage of forced expiratory volume in the first second of expiration for predicted values.
- a The tumor, node, and metastasis (TNM) stage was performed according to the International Association for the Study of Lung Cancer eighth edition staging criteria.
Long-term survival
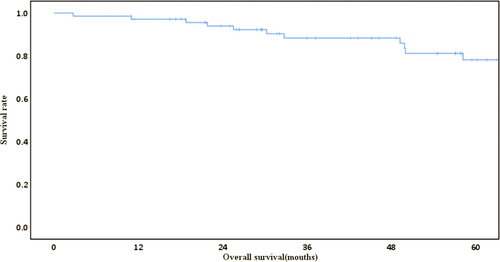
The median follow-up was 50 months (range, 3–120). During follow-up, 11 patients died, 10 deaths were due to lung cancer, and one was due to malnutrition. The 5-year overall survival was 78.2% (Figure 1).
DISCUSSION
We found that secondary ipsilateral VATS was feasible and safe for lung cancer patients who had a prior pulmonary resection provided the surgeon had skilled technology. In addition, the oncological efficacy was satisfactory. There are two principal situations for secondary ipsilateral surgery; locoregional recurrence and second primary lung cancer, the treatment for which varies. Even if surgery is indicated, open surgery remains an alternative. For many years, our institution has been committed to VATS lung resection technology, and most operations are indeed performed by VATS. However, investigators have not focused on the feasibility and safety of VATS for ipsilateral lung cancer after pulmonary resection.6
With advances in surgical techniques and medical devices, VATS has become a widely accepted technique and is recommended by the National Comprehensive Cancer Network as the preferred surgical approach for early-stage non-small cell lung cancer (NSCLC). VATS has many benefits compared with traditional thoracotomy, such as smaller incision, milder postoperative pain, shorter hospital stay, less inflammatory response, faster recovery, and higher patient satisfaction7; secondary VATS also has such merits. In patients with prior pulmonary resection, issues such as pleural adhesions and distorted hilum anatomical structure are significant challenges for secondary VATS surgeons. The median length of time for the secondary surgery in this study was 120 min, and the median blood loss was 50 ml. Ten patients had converted thoracotomy due to severe pleural adhesions, and two patients had reinsertion of chest tube due to pneumothorax. No patient died within 30 days. We also found that high preoperative CCI and severe pleural adhesions were independent risk factors for complications. All these characteristics indicated that secondary VATS was feasible and safe.
Pleural adhesion is an abnormal fibrous connection formed during the natural repair process that originates from the injury of the first operation. Pleural adhesion can lead to unclear tissue boundaries, increased intraoperative bleeding, and a chance of injury to vital organs in the chest. Although surgeons are experienced in such complicated operations, they must be prepared for unexpected situations. When a high-risk intraoperative event occurs, a surgeon cannot hesitate to switch surgical methods. In this study, 10 patients (14.3%) had converted thoracotomy in secondary surgery, which was better compared with the 20% previously reported.8-11 In addition, the postoperative complication rate in this study was lower compared with previous studies, such as 29% reported by Hamaji et al.,12 34.3% in a study by Yang et al.,13 and 36.5% reported by Abid et al.14
The most common complication of secondary pulmonary resection was air leakage, which agreed with the findings of Sun et al.6 The main cause of air leakage was intraoperative adhesion. At present, there is no uniform standard for the evaluation of pleural adhesion. We defined and analyzed the degree of pleural adhesion, which provides guidance for the study of pleural adhesion. We suggest that, in addition to careful treatment of pleural adhesions, an effective solution to prevent air leakage is to carefully check the area and repair lung tissue with prolene sutures. Ikeda et al.15 used free pericardial fat pads as a sealant to control air leakage, and the mean time to postoperative air leakage was 1.05 ± 1.84 days.16 Using the TachoSil technique, Marta et al.17 reported a reduction in the duration of persistent postoperative air leakage.
In this study, univariate analysis showed that smoking, surgical time ≥120 min, blood loss ≥100 ml, conversion to thoracotomy, high CCI, and severe pleural adhesions were significantly associated with postoperative complications. Multivariate analysis showed that high CCI and severe pleural adhesions were associated with a high number of postoperative complications. Yang et al.13 reported that patients aged >70 years were prone to postoperative complications in the second surgery. Hamaji et al.12 found that poor pulmonary function was an independent risk factor for postoperative complications after second surgery for metachronous MPLC. In addition to careful in-operative management, more aggressive preoperative evaluation and screening of patients are needed to deal with intraoperative adhesions. Nagatani et al.18 used dynamic ventilation CT for preoperative evaluation of localized pleural adhesions with a sensitivity of 82.5%. Rosen et al. used the National Cancer Database to investigate surgically treated patients who had NSCLC19; they found that a high CCI was associated with high rates of postoperative adverse events and longer postoperative hospital stays. Wang et al.16 reported that patients with CCI ≥2 had higher perioperative mortality after surgery compared with patients with CCI <2. Therefore, for patients with more comorbidities and poor baseline conditions, careful history-taking, physical examination, and a rigorous preoperative evaluation are required to guide the formulation of a surgical treatment strategy.
In addition to the feasibility and safety of surgery, what is more gratifying is that the 5-year overall survival after the secondary operation in our group of patients was as high as 78%, which was paramount to the survival of those after the first operation and should be superior to those receiving other nonsurgical treatment methods. Leroy et al.20 showed that the cumulative risk of second primary lung cancer 10 years after the first surgery was 20.2%. Han and colleagues21 reported 102 (4.6%) local oligometastatic recurrences 10 years after 2230 people underwent surgery. The treatment for patients with locoregional recurrence or second primary lung cancer is still controversial. Because of the difficulty of secondary surgery and poor lung function, traditionally, oncologists have used nonsurgical methods. As the lung cancer treatment field continues to evolve, reoperation after first surgery has become a significant option. The effectiveness of reoperation has been progressively demonstrated in retrospective reports. Zhou et al.22 confirmed that patients who underwent repeat surgery had better oncological efficacy compared with patients treated with nonsurgical methods. Hattori and colleagues23 reported that the 3-year overall survival after surgery for 104 ipsilateral second primary lung cancers was 80.1%, which indicated that secondary operation could be an option for second pulmonary disease. The satisfactory survival in our study also demonstrates the necessity of secondary operation for lung cancer patients with a prior surgery.
In conclusion, the results of this study proved that secondary VATS pulmonary resection was feasible and safe for patients with ipsilateral second lung cancer. The pleural adhesion caused by the first surgery is the challenge for the secondary operation. Strict preoperative evaluation, careful intraoperative maneuver, and timely conversion to thoracotomy when needed are the guarantee of safe operation. The oncological results also proved that ipsilateral VATS pulmonary resection had long-term survival benefit.
The limitations of this study are that the investigation was a retrospective study from a single center, a condition which inevitably has selection bias. The large operation volume of our hospital and the rich experience of our surgeon may also have restricted the popularization of this study. We expect more prospective multicenter studies to confirm our results.
AUTHOR CONTRIBUTIONS
Dr. Lunxu Liu was responsible for the study design and quality control, Lei Chen was responsible for the data collection, data analysis and drafting, Zhenyu Yang and Ruichen Cui was responsible for data collection.
ACKNOWLEDGMENT
This study was supported by the Sichuan Provincial Department of Science and Technology Program of Sichuan Province (no. 2021YFS0024).
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.




