Real-world treatment and prognostic factors for survival in ALK+ non-small cell lung cancer (NSCLC) patients with brain metastases in China
Na Li, Mingying Xie, Xiaoqing Yu and Yun Fan contributed equally to this study.
Abstract
Background
To explore the efficacy and prognostic factors of different treatment modalities on anaplastic lymphoma kinase (ALK)+ non-small cell lung cancer (NSCLC) patients with brain metastases (BMs).
Methods
A total of 86 patients were enrolled into the study. They were divided into two cohorts based on their history of treatment with ALK tyrosine kinase inhibitors
(ALK-TKIs) prior to the incidence of BMs. ALK-TKI-naïve patients with BMs were included in cohort 1 (n = 59); patients who developed BMs after ALK-TKIs treatment were enrolled in cohort 2 (n = 27). Prognostic factors related with overall survival (OS) when treated with ALK-TKIs were assessed in multivariable analysis.
Results
With a median follow-up of 41.8 months, the median OS was 34.8 months. In cohort 1, the OS, intracranial progression-free survival (iPFS), and progression-free survival (PFS) were 38.7 months (95% CI: 23.3 to 54.1), 18.5 months (95% CI: 9.6 to 27.4), and 19.1 months (95% CI: 13.7 to 24.5), respectively. Significantly improved OS and iPFS were noted in those patients in which second-generation ALK-TKIs versus crizotinib were initiated (OS: not reached vs. 29.0 months, p = 0.040; iPFS: 22.8 vs. 11.9 months, p = 0.035). In cohort 2, patients who experienced BMs as a result of the treatment failure of ALK-TKIs had a median OS of 27.1 months. Considerable duration of stable disease in patients with measurable BMs was observed (iPFS: 11.5 months, 95% CI: 4.4 to 18.6; PFS: 12.2 months, 95% CI: 3.2 to 21.1).
Conclusion
Second-generation ALK-TKIs further improved the duration of intracranial response and survival in ALK+ NSCLC patients with BMs in a real-world setting. The potent intracranial efficacy of second-generation ALK-TKIs might generate the lowered urgency of local treatment.
INTRODUCTION
The anaplastic lymphoma kinase (ALK) gene accounts for about 3%–7% of non-small cell lung cancer (NSCLC) cases and is common in younger, never/light smokers and patients with adenocarcinoma of the lung.1, 2 It has been demonstrated that first-generation ALK tyrosine kinase inhibitors (TKIs) significantly prolong progression-free survival (PFS) and improve prognosis compared with platinum-based chemotherapy in metastatic ALK+ NSCLC patients.3, 4 However, a significant increase in the incidence rate of brain metastases (BMs) over time has been observed despite the important survival benefit achieved from ALK-TKIs therapy.5, 6 Patients identified with BMs at the time of diagnosis account for approximately 25% of NSCLC cases, and BMs may develop in a higher proportion during treatment of the disease, which is probably a result of the prolonged survival associated with newer therapies together with the improvement of neurological imaging techniques.5, 7 Crizotinib is the first small molecule inhibitor of ALK tyrosine kinases used in the treatment of advanced ALK+ NSCLC patients and has significantly improved the median PFS and objective response rates (ORR) compared to standard chemotherapy.4, 8, 9 Nevertheless, up to 70% of ALK+ NSCLC patients develop BMs during treatment,10, 11 which mainly results from its ineffective penetration of the blood–brain barrier (BBB).11 Higher intracranial control rates were reported using newer-generation ALK-TKIs in several clinical trials, including aletinib, ensartinib, ceritinib, brigatinib, and loratenib.12-15 Patients with BMs are, however, underrepresented in clinical trials. Thus, their efficacy in patients with BMs is still not well illustrated. Data on the prognosis of survival in a real-world setting for ALK+ NSCLC patients with BMs in China are currently lacking. Therefore, we conducted this retrospective study to explore the efficacy and prognostic factors of different treatment modalities on ALK+ NSCLC patients with BMs.
METHODS
Patients
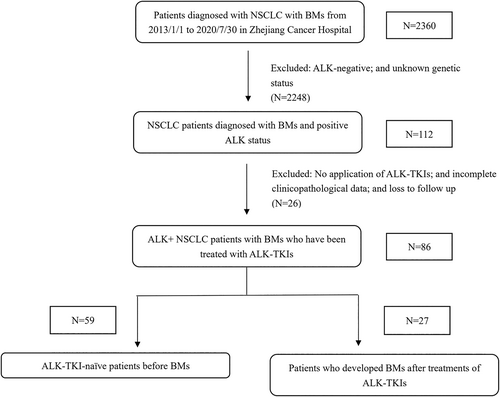
A total of 2360 patients with NSCLC and BMs were identified in Zhejiang Cancer Hospital from January 2013 to July 2020 through electronic medical records. Among them, patients who met the following criteria were enrolled in this study: (1) histologically or cytologically confirmed pulmonary malignancy; (2) brain metastases confirmed by cranial magnetic resonance imaging (MRI) or computed tomography (CT); (3) positive ALK status determined by Ventana anti-ALK (D5F3) immunohistochemistry (IHC), fluorescence in situ hybridization (FISH), real-time reverse transcription polymerase chain reaction (RT-PCR), or next-generation sequencing (NGS); (4) age 18 years old or older; (5) have been treated with ALK-TKIs; and (6) complete follow-up records available. The final eligible cohort encompassed 86 NSCLC patients diagnosed with BMs and positive ALK status. The patient selection process is shown in Figure 1. Due to the retrospective setting of our analysis, informed consent from the patients was not required.
Data collection and follow-up
All enrolled patients had received ALK-TKIs. OS was defined from the point of BMs diagnosis to the date of either death or the last follow-up. Intracranial progression-free survival (iPFS) and PFS were calculated from the beginning of ALK-TKI treatment to the first recorded evidence of progression by imaging, the time of last imaging record, or the date of death, as applicable. The intracranial objective response rate (iORR), including complete response (CR) and partial response (PR), was based on the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). Deferral brain radiotherapy (RT) was defined as more than 1 month from the diagnosis of BM to the receipt of intracranial RT. The clinical characteristics, age, gender, smoking history, extracranial metastases (ECM), number of BMs (1–3 or more than 3), BM symptoms, gene mutation status, and other clinicopathological data were collected. Patients enrolled in this study were divided into two cohorts based on their ALK-TKI treatment history prior to the incidence of BMs. ALK-TKI-naïve patients with BMs were included in cohort 1; cohort 2 included patients who developed BMs with or without extracranial metastases after treatment with ALK-TKIs. All patients were stratified according to stratification disease-specific graded prognostic assessment (DS-GPA) criteria.16 The data cutoff date was April 1, 2022. Demographic data of all patients are illustrated in Table 1.
| Baseline characteristic | Cohort 1, N = 59 | Cohort 2, N = 27 |
|---|---|---|
| Age (median) | 55 (range 30, 81) | 51 (range 25, 64) |
| Sex (%) | ||
| Male | 23 (38.9%) | 13 (48.1%) |
| Female | 36 (61.1%) | 14 (51.9%) |
| Smoking history (%) | ||
| Smoker | 16 (27.1%) | 8 (29.6%) |
| Nonsmoker | 43 (72.9%) | 19 (70.4%) |
| Performance status (%) | ||
| KPS < 80 | 16 (27.1%) | 4 (14.8%) |
| KPS ≥ 80 | 43 (72.9%) | 23 (85.2%) |
| Histology subtype (%) | ||
| Adenocarcinoma | 53 (89.8%) | 27 (100%) |
| Nonadenocarcinoma | 6 (10.2%) | 0 (0%) |
| Fusion types (%) | ||
| EML4-ALK | 48(81.4%) | 24(88.9%) |
| Complex variants involving ALK | 10(16.9%) | 3(11.1%) |
| Non-EML4 ALK | 1(1.7%) | 0(0.0%) |
| BM number (%) | ||
| 1–3 | 24 (40.7%) | 10 (37.0%) |
| >3 | 35 (59.3%) | 17 (63.0%) |
| Symptoms related to BMs (%) | ||
| Yes | 28 (47.5%) | 16 (59.3%) |
| No | 31 (52.5%) | 11 (40.7%) |
| ECM (%) | ||
| Yes | 51 (86.4%) | 24 (88.9%) |
| No | 8 (13.6%) | 3 (11.1%) |
| Intracranial local therapy | ||
| Yes | 42 (71.2%) | 19 (70.4%) |
| No | 17 (28.8%) | 8 (29.6%) |
| Local therapies | ||
| WBRT | 32 (76.2%) | 11 (57.9%) |
| SRS | 10 (23.8%) | 8 (42.1%) |
| Timing of local therapies | ||
| Synchronous | 22 (52.4%) | 5 (26.3%) |
| Deferral | 20 (47.6%) | 14 (73.7%) |
- Abbreviations: EML4, enchinoderm microtubule associated ptotein-like 4; ALK, aBMs, brain metastasis; ECM, extracranial metastases; KPS, Karnofsky performance score; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Statistical analysis
The primary endpoint was OS, which is expressed in terms of 95% CI. The secondary endpoint included PFS, iPFS, ORR, and iORR. We applied the univariable and multivariate analyses to estimate the OS, PFS, iPFS, and prognostic factors. Time to intracranial progression and time to treatment failure were evaluated using Kaplan–Meier curves. Prognostic factors were assessed with multivariate Cox proportional hazard models. The log-rank test was used to assess the differences between groups and 95% confidence interval (CIs). All analyses were conducted using SPSS version 26.0. Survival curves were plotted using GraphPad Prism 9, and p < 0.05 was considered to indicate statistically significant differences.
RESULTS
Patient demographics
A total of 86 patients treated from January 2013 to July 2020 were finally considered eligible for our study (cohort 1: n = 59; cohort 2: n = 27). The baseline clinical features of the patients were detailed in Table 1. The median age was 55 years old, with age ranging from 25 to 81 years old. The cohort was divided into two groups according to whether treatment with ALK-TKIs had occurred before or after the development of BMs. A total of 59 patients were diagnosed with BMs before the initiation of ALK-TKIs (cohort 1), whereas 27 patients were found to have developed BMs during the treatment of ALK-TKIs (cohort 2). In each cohort, 58 (98.3%) and 24 (82.8%) patients had measurable brain lesions, while 28 (47.5%) and 16 (59.3%) patients had symptoms related to BMs. Extracranial metastases were found in 51 and 24 patients in cohorts 1 and 2, respectively. Of the initial cohort of 86 patients, over half (n = 60, 69.8%) were under 60 years old, 50 (58.1%) patients were female, and 24 (27.9%) had a history of smoking. Furthermore, lung adenocarcinoma accounted for the majority of patients (n = 80, 93.0%), while 7.0% were diagnosed with NSCLC of other pathological types. Under the real-world background, the ALK-positive status of patients was confirmed using different genetic testing methods at diagnosis: 37 (43.0%) with next-generation sequencing (NGS), 38 (44.2%) with immunohistochemistry (IHC), and 11 with polymerase chain reaction (PCR). In the 37 patients who were profiled by NGS, pure EML4-ALK fusion is the frequent variant occupying 62.2% (23/37), as well as compound variants involving EML4-ALK 35.1% (13/37) and non-EML4-ALK variants 2.7% (1/37). We then checked the coalterations of other genes along with ALK fusion. Tumor protein p53 (TP53) mutation was found in six patients and EGFR D1012H mutation in two patients. The spectrum of other genomic coalterations contained KRAS, PTEN, EGFR A289V, SOX2 S242L, EGFR copy number amplification and ALK 19 exon breakage rearrangement. Most patients (n = 52, 60.5%) had more than three BM lesions. A total of 61 (70.9%) patients received local brain RT, including whole-brain radiotherapy (WBRT) and/or stereotactic radiosurgery (SRS). A total of 34 (55.7%) patients underwent deferred local therapy. Notably, in cohort 1, 71.2% of patients with BMs received RT, including 26 patients who received crizotinib plus RT.
Survival outcomes
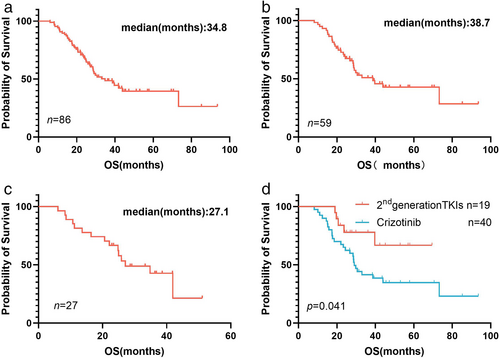
With a median follow-up of 41.8 months (95% CI: 37.2 to 46.4), the OS after BMs was 34.8 months (95% CI: 24.3 to 45.4) (Figure 2(a)). In cohort 1, the OS, iPFS, and PFS were 38.7 months (95% CI: 23.3 to 54.1), 18.5 months (95% CI: 9.6 to 27.4), and 19.1 months (95% CI: 13.7 to 24.5), respectively (Figures 2(b) and 3(a, d)). The intracranial response was estimable in 58 patients, and the extracranial response was measurable in 51 patients. An iORR of 74.1% (95% CI: 61.0 to 84.7) and ORR of 78.4% (95% CI: 64.7 to 88.7) were reported (Table 2). In total, patients who had received RT showed a similar 24-month OS rate to those treated without RT (69.0% (95% CI: 54.5 to 83.6) vs. 62.6% (95% CI: 26.5 to 79.4), p = 0.404). We further explored the administration timing of RT in patients with BMs. It was notable that patients experiencing deferral RT had longer OS compared with those who underwent synchronous RT (OS: 73.2 months vs. 29.0 months, 95% CI: 0.264 to 1.061, p = 0.055). In addition, when comparing patients treated with SRS with those who adopted WBRT, no association was found with OS (p = 0.229).

| Response | Extracranial efficacy | Intracranial efficacy | ||
|---|---|---|---|---|
| Cohort 1, N = 51 | Cohort 2, N = 21 | Cohort 1, N = 58 | Cohort 2, N = 24 | |
| PR | 40 (78.4%) | 11 (52.4%) | 29 (50.0%) | 8 (33.3%) |
| CR | 0 (0.0%) | 0 (0.0%) | 14 (24.1%) | 7 (29.2%) |
| SD | 8 (15.7%) | 7 (33.3%) | 13 (22.4%) | 7 (29.2%) |
| PD | 3 (5.9%) | 3 (14.3%) | 2 (3.4%) | 2 (8.3%) |
| ORR | 40 (78.4%) | 11 (52.4%) | 43 (74.1%) | 15 (62.5%) |
- Abbreviations: ALK-TKIs, anaplastic lymphoma kinase tyrosine kinase inhibitors; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease; ORR, objective response rate.
Of the 59 patients in cohort 1, 40 (67.8%) patients initially received crizotinib and 19 (32.2%) initially received second-generation ALK-TKIs after BMs, including alectinib (n = 15), brigatinib (n = 2), ensartinib (n = 2). Between the two groups above, the iORR and ORR were 69.2% (95% CI: 52.4 to 83.0) versus 84.2% (95% CI: 60.4 to 96.6) and 77.8% (95% CI: 61.8 to 89.9) versus 80.0% (95% CI: 51.9 to 95.7), respectively. Additionally, significantly improved OS and iPFS were observed in patients who underwent treatment with second-generation ALK-TKIs, although there was no difference in extracranial efficacy (OS: not reached vs. 29.0 months, 95% CI: 0.2 to 0.8, p = 0.040; iPFS: 22.8 vs. 11.9 months, 95% CI: 1.1 to 4.3, p = 0.035; PFS: 14.4 vs. 23.4 months, 95% CI: 0.4 to 1.8, p = 0.095) (Figures 2(d) and 3(c, f)). For patients initially treated with crizotinib, the 26 (65.0%) who received combined RT tended to have longer OS and iPFS than those in the TKI-alone group (OS: 31.8 vs. 26.8 months, 95% CI: 0.3 to 1.4, p = 0.177; iPFS: 14.2 vs. 11.4 months, 95% CI: 0.3 to 1.4, p = 0.191).
In cohort 2, 27 patients experienced BMs as a result of treatment failure of ALK-TKIs, with a median OS of 27.1 months (95% CI: 14.1 to 40.1) (Figure 2(c)), iPFS of 11.5 months (95% CI: 4.4 to 18.6) (Figure 3(b)), and PFS of 12.2 months (95% CI: 3.2 to 21.1) (Figure 3(e)). Moreover, we observed an ORR of 52.4% (95% CI: 29.8 to 74.3) and iORR of 62.5% (95% CI: 40.6 to 81.2) (Table 2). Of these, 25 patients progressed to BMs following treatment with crizotinib. Only two patients developed BMs after alectinib resistance. Among the 25 crizotinib-resistant patients with BMs, 19 patients sequentially received second-generation ALK-TKIs, and the 24-month OS rate was 73.7% (95% CI: 51.9 to 95.5).
We conducted univariate survival analysis based on various factors to exclude the influence of other variables on OS (Table 3). We noted that the patients with asymptomatic BMs had superior survival (not reached vs. 28.8 months, p = 0.013) (Figure 4(a)), while the presence of second-generation ALK-TKIs treatment did not impact the survival outcomes (p = 0.138) (Figure 4(b)). Patients with >3 brain lesions had similar outcomes to those with fewer brain lesions (p = 0.431) (Figure 4(c)). No statistically significant differences in survival were observed between patients with or without ECM (33.0 months vs. not reached, p = 0.403) (Figure 4(d)). Patients with higher Karnofsky performance scores (KPS) (≥80) showed superior survival outcomes (p < 0.0001) (Figure 4(e)). Next, for the 37 patients profiled with NGS, we further analyzed the association between different ALK variants and clinical outcomes. The compound ALK variants group tended to have better OS versus pure EML4-ALK variant group (not reached vs. 28.57 months, p = 0.038).
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| 95% CI | p-value | 95% CI | HR | p-value | |
| Second-generation ALK-TKIs | 0.26–1.36 | 0.138 | 0.21–8.68 | 0.846 | 0.654 |
| BM-number | 0.71–2.28 | 0.431 | 0.53–1.86 | 0.994 | 0.985 |
| Symptomatic BMs | 1.17–3.74 | 0.013 | 0.76–2.81 | 1.461 | 0.257 |
| ECM | 0.65–3.66 | 0.403 | 0.48–3.86 | 1.365 | 0.558 |
| KPS (<80) | 0.10–0.54 | <0.0001 | 1.93–7.32 | 3.761 | <0.0001 |
| GPA (>1.5) | 1.27–4.10 | 0.012 | – | – | – |
| Modified GPA (>1) | 0.31–0.98 | 0.039 | – | – | – |
- Abbreviations: ALK-TKIs, anaplastic lymphoma kinase tyrosine kinase inhibitors; BMs, brain metastasis; CI, confidence interval; ECM, extracranial metastasis; GPA, graded prognostic assessment; HR, hazard ratio; KPS, Karnofsky performance score.
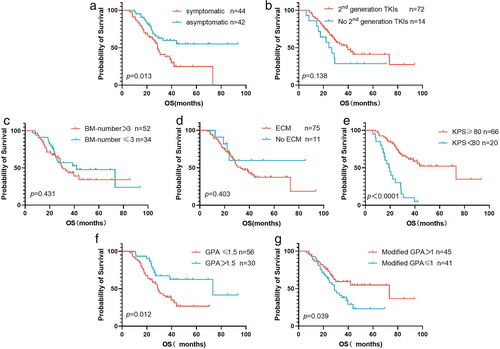
According to the DS-GPA index from lung cancer, patients with GPA scores >1.5 had a longer OS of 73.2 months compared with the 29.0 months of patients who had scores ≤1.5 (p = 0.012) (Figure 4(f)). We also applied the modified Lung-molGPA index to further validate the feasibility of this observation. Our study showed prognostic differences between patients with modified GPA scores of >1 and ≤1 (73.2 vs. 28.7 months, p = 0.039) (Figure 4(g)).
Additionally, we included the presence or absence of second-generation ALK-TKI treatment, numbers of brain lesions, KPS, ECM, and the symptoms related to BMs as independent prognostic factors in the Cox regression model (Table 3). Prognostic factors for the longer OS, when treated with ALK TKIs, were higher KPS scores (p < 0.0001) in multivariable analysis (Table 3).
DISCUSSION
Currently, the optimal modality of subsequent treatment after BMs in advanced ALK+ NSCLC patients is poorly characterized, and evidence-based guidance is lacking on the real-world question of how to optimally manage patients with symptomatic or unstable BMs. This study explored the clinical experience and survival outcomes of ALK+ NSCLC patients with BMs exposed to ALK-TKI treatment in a real-world setting in China.
In our study, patients with ALK+ NSCLC and BMs had a median OS of 34.8 months under sequential treatment with multiple generations of ALK-TKIs in a real-world setting. Notably, the patients in cohort 1, who had been diagnosed with BMs before ALK-TKI treatment, could live for over 3 years, with a median OS of 38.7 months. The favorable survival outcome was mostly derived from the utilization of second/third-generation ALK-TKIs. Based on the data of prior clinical trials, ALK-TKIs are recommended as the standard first-line care for patients with advanced ALK+ NSCLC.13, 17-19 However, the central nervous system (CNS) is a common site of progression in patients treated with crizotinib as a result of dismal intracranial efficacy.10 In the present study, we noted that patients who experienced BMs due to ALK-TKI treatment failure achieved an OS of 27.1 months. Similar results were also observed in a previous clinical trial, in which the median OS reached 28.5 months.20 Another retrospective study by Xing et al. reported an OS of 28.5 months in patients developing BMs during ALK-TKI treatment.21 To improve intracranial efficacy, several second-generation ALK-TKIs were developed that were then substantiated to have a potent antitumor curative effect in subsequent clinical trials, both in first-line cases and in those that had previously received and were found to be resistant to ≥1 ALK inhibitors.22
According to the National Comprehensive Cancer Network (NCCN) guidelines,23 second-generation ALK inhibitors are recommended as a first-line therapy in patients with BMs in clinical practice due to their better BBB penetration. However, it is not yet clear whether alectinib is associated with better OS versus crizotinib in the first-line setting, because of the fact that the updated results reported in the J-ALEX study differed from those of the previous ALEX study.24 Here, we compared the survival outcomes of patients with BMs initially receiving second-generation TKIs versus those receiving crizotinib after BMs. The administration of initial second-generation TKIs resulted in a significant improvement in OS (p = 0.040) and iPFS (p = 0.035). Preclinical studies also supported the antitumor activity of next-generation TKIs within brain lesions.25 Prior to our study, multiple studies have explored the efficacy of next-generation ALK-TKIs in patients with BMs at baseline.26-29 Regarding the ALEX study,30 alectinib showed robustly better iPFS results in patients with BMs, which was consistent with our findings. In the ASCEND series studies,19, 31, 32 the superior CNS control of ceritinib was also noted. Furthermore, lorlatinib demonstrated remarkable intracranial antitumor activity as post-line treatment in the CROWN trials.33 Despite having patients with unstable BMs enrolled in cohort 1, the iPFS of 22.8 months in patients treated with second-generation ALK-TKIs was similar to the intracranial control time of these clinical trials. The possible reasons for this phenomenon are as follows. In a real-world setting, patients prefer to strengthen intracranial disease control using repeated brain interventions, which are conducive to extending intracranial control time. Lin et al. suggested the robust intracranial efficacy of alectinib in ALK+ NSCLC patients with baseline, symptomatic BMs, with a median time to CNS progression of 18.6 months,28 lower than the iPFS described in cohort 1. The main reason is that only patients with symptomatic or large (≥1 cm) BMs were allowed to enroll in the study, while our study included asymptomatic patients and patients with lower tumor burden. Another study addressed the substantial intracranial activity of alectinib in patients with BMs irrespective of their brain condition and history of ALK-TKI treatment.26 Admittedly, the second-generation ALK-TKIs demonstrated higher intracranial efficacy and impressive survival benefits in our analysis, suggesting the superiority of second-generation ALK-TKIs over crizotinib.
For ALK+ patients with BMs, interdisciplinary collaboration could improve their quality of life. Some retrospective studies have investigated the value and optimal intervention timing of intracranial RT plus TKIs in oncogene-driven patients with BMs. In our study, we noted that the administration of RT treatment (including SRS and/or WBRT) resulted in relatively prolonged survival. However, the most appropriate sequence or combination of ALK-TKIs plus intracranial RT was poorly illustrated. In cohort 1, we noted that 71.2% of patients with BMs received RT and these patients exposed to local RT had longer survival. In terms of CNS efficacy, the intracranial control time in patients treated with crizotinib in cohort 1 was higher than the PFS of 7.4 months reported in the ALEX study.30 In addition, in our study, crizotinib demonstrated a better therapeutic effect in CNS lesions, with 69.2% of iORR in our analysis and 40.0% in the ALEX study.34 The better intracranial response is probably ascribed to the combination of cranial RT, since patients with BMs are more likely to adopt the combined regimen to achieve a better intracranial response. Another possible reason is that crizotinib showed some efficacy in patients with BMs. Although the application of intracranial radiation could strengthen local disease control within CNS, it is associated with a greater risk of decline in neurocognitive function.5, 35 Therefore, identifying the population benefiting from RT treatment warrants further study. A recent study reported an association of patients exposed to first-line RT for BMs with longer intracranial control time compared to EGFR/ALK-TKIs alone,36 which is consistent with the results observed in our study. In their systematic review and meta-analysis of 30 studies, Singh et al. noted similar OS results between RT+ EGFR/ALK-TKIs, EGFR/ALK-TKIs alone, and RT alone.37 The ACEND-7 study reported the active intracranial response of ceritinib in patients with BMs at baseline; however, prior exposure to brain RT did not seem to influence the intracranial response.31 Regarding the utilization of CNS-penetrating ALK-TKIs, second-generation ALK-TKIs showed an equivalently active intracranial response without combined local RT. Therefore, intracranial RT was demonstrated to be beneficial for brain disease control, especially in patients treated with crizotinib.
In the present study, we also analyzed the efficacy of second-generation ALK-TKIs in crizotinib-pretreated patients with BMs in a real-world setting. It is noteworthy that crizotinib-pretreated patients who received second-generation ALK-TKIs as sequential treatment experienced promising intracranial disease control. The active intracranial efficacy of second-generation ALK-TKIs in our analysis is supported by previous clinical results. Based on the data of ALEX focusing on CNS efficacy,34 85.7% of CNS ORR was observed in patients with measurable baseline BMs, which was comparable to that in our cohort 1. In a retrospective study by Professor Yang,27 a CNS response of alectinib as first-line or sequential treatment occurred in 95.6% and 82.4% of patients with measurable CNS lesions, respectively. In general, our results substantiated the robust CNS potency of second-generation ALK-TKIs irrespective of the therapy line in the real-world.
The DS-GPA index has been widely used to assess the prognosis of patients with BMs from lung cancer, and several clinical studies have validated its practical application.38, 39 Based on the original DS-GPA index, Pawl et al. updated the Lung-molGPA index by incorporating gene mutation status.40 The DS-GPA index and Lung-molGPA confirmed that patients with higher scores had a better prognosis. The latter was the more accurate prognostic prediction for oncogene-addicted patients with BMs. In our analysis, there were no significant differences in OS based on the number of BM lesions. Likewise, the number of BMs showed no prognostic value in our previous study.41 The conclusion might be related to the administration of systematic treatment, especially given the superior intracranial response of TKIs. The applicability of the DS-GPA model and modified Lung-molGPA index were verified in the present study, suggesting that they are still valid tools for predicting prognosis in clinical practice.
Our study is limited by its single-institutional retrospective setting. Given the relatively small sample size, our results should be treated cautiously. In addition, since the majority of the population in our study received crizotinib in the first setting, data on CNS progression related to alectinib are relatively lacking. Further, lorlatinib is not yet available in China, rendering the analysis on treatment data difficult. Another limitation of our study is that quality-of-life or neurocognitive follow-up data from patients were not available, which prevented analysis of these factors.
In conclusion, the positive intracranial and extracranial effect of second-generation ALK-TKIs is attributable to the impressive gains in survival and higher intracranial activity, which makes them a preferred option as first-line or beyond crizotinib-resistant treatment in ALK+ NSCLC patients with BMs. There is a trend toward prolonged survival in crizotinib-treated patients with the application of intracranial RT. For crizotinib-pretreated patients with BMs, the administration of second-/third-generation ALK-TKI treatment alone should be considered. However, our conclusions should be treated with caution due to the limited sample size of our study.
AUTHOR CONTRIBUTIONS
Na Li, Mingying Xie, Xiaoqing Yu and Yun Fan contributed equally to this paper. Conceptualization: Yun Fan, Xiaoqing Yu, Na Li; Methodology: Yun Fan, Xiaoqing Yu; Formal analysis and investigation: Na Li, Mingying Xie and Zichao Zhou; Writing - original draft preparation: Na Li, Mingying Xie; Writing - review and editing: Yun Fan, Xiaoqing Yu; Resources: Na Li, Jiamin Sheng and Mingying Xie; Supervision: Yun Fan.
ACKNOWLEDGMENTS
The follow-up data of patients from the Follow-up Office of Zhejiang Cancer Hospital is gratefully acknowledged.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




