The survival after discontinuation of EGFR-TKIs due to intolerable adverse events in patients with EGFR-mutated non–small cell lung cancer
Funding information: This work was supported by grants from Linkou Chang-Gung Memorial Hospital (CMRPG3M0971 to C-E.W.) This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abstract
Background
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are standard treatments for advanced non–small cell lung cancer (NSCLC) patients harboring the EGFR mutation. Patients experiencing intolerable adverse events (AEs) would discontinue EGFR-TKIs. This study aimed to evaluate the impact of intolerable AEs and subsequent treatment on the survival of patients who discontinued EGFR-TKIs.
Patients
The data of advanced NSCLC patients treated with EGFR-TKIs as frontline treatment at Chang Gung Memorial Hospitals from June 2014 to March 2018 were retrospectively reviewed.
Results
A total of 2190 patients were enrolled and treated with frontline EGFR-TKIs. In August 2021, 114 (5.2%) patients experienced intolerable AEs requiring discontinuation of EGFR-TKIs. The time median of EGFR-TKIs discontinuation was 2.56 months. Age >65 years, females, body weight, and body surface area were associated with the occurrence of intolerable AEs for patients treated with afatinib. Patients experiencing skin/paronychia/mucositis and abnormal liver function test had favorable survivals results. Patients who received subsequent EGFR-TKIs treatment, experienced better progression-free survival (PFS), total PFS (from frontline line EGFR-TKIs), and overall survival (OS) compared to patients receiving chemotherapy or no treatment. Patients undergoing subsequent EGFR-TKIs had better total PFS (median, 14.9 vs. 11.3 months, p = 0.013) and OS (median, 31.3 vs. 20.1 months, p = 0.001) than patients who did not discontinue because of AEs. Favorable OS was validated by propensity score matching.
Conclusion
Patients experiencing intolerable AEs during EGFR-TKI treatment should consider switching to an alternative EGFR-TKI, which increase the survival results as compared to those patients who did not experience intolerable AEs.
INTRODUCTION
Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are the standard of care in non–small cell lung cancer (NSCLC) patients harboring the EGFR mutation.1-4 Adverse events (AEs) frequently occurred during EGFR-TKI treatment, and some patients required dose reduction or discontinuation because of intolerable, severe AEs. In the LUX-Lung 7 study, phase 2b trials compared afatinib and gefitinib in patients with EGFR-mutated NSCLC. Dose reductions because of AEs were undertaken mostly with afatinib (42%) rather than gefitinib (2%), and 6% of patients in each group discontinued treatment because of drug-related AEs.3 In the FLAURA study, comparing osimertinib with standard EGFR-TKIs (gefitinib or erlotinib) in patients with EGFR-mutated NSCLC, the discontinuation rates were 13% and 18% for osimertinib and standard EGFR-TKIs, respectively.5
Dose reduction did not compromise the therapeutic efficacy from post hoc analysis of six trials of afatinib in LUX-Lung 36 and may indicate better survivals than the experience of dacomitinib in ARCHER 1050.7 Similar findings were found in real-world experiences.8-11 The above studies show that the patients with AE requiring dose modifications had higher baseline plasma concentrations of EGFR-TKI than those without severe AE, and dose modifications for these patients could achieve compatible plasma concentrations to those patients without severe AE.6, 7 Therefore, the clinical efficacy may not be influenced by dose modification because of intolerable AEs.
However, the survival outcomes for the patients who discontinued EGFR-TKIs because of AEs and the association between types of AEs and survivals were not well-known. Few studies specifically evaluated the impact of discontinuation of EGFR-TKIs on survival outcomes. Therefore, the current study aimed to investigate the causes of EGFR-TKI discontinuation, the factors associated with EGFR-TKIs discontinuation and their impact on survival outcomes.
MATERIALS AND METHODS
Data collection
Patients eligible for the current study were obtained from the Chang Gung Research Database (CGRD).12 CGRD is an electronic database with medical records from all branches of Chang Gung Memorial Hospital (CGMH) in Taiwan. The data of patients were collected from the cancer registry system of CGMH.
Patients and their clinicopathological features
Patients with advanced NSCLC cancer harboring the EGFR mutation (either the exon 19 deletion or the L858R mutation) treated with EGFR-TKIs as frontline treatment during June 2014 and March 2018 were enrolled in the study. The clinicopathological features were recorded, including age, gender, performance status (PS), tumor histology, smoking history, EGFR mutation (exon 19 deletion or L858R mutation), body weight (BW), body surface area (BSA), body mass index (BMI), metastatic sites of NSCLC, clinical response to EGFR-TKIs and chemotherapy, and subsequent treatment (chemotherapy, EGFR-TKIs). The last follow-up time point in the study was August 2021.
Tumor response, survivals, and statistical analysis
The clinical tumor response was assessed based on the Response Evaluation Criteria in Solid Tumors 1.1 criteria, and the detailed definition of tumor response, progression-free survival (PFS) and overall survival (OS) were referred to in our previous study.8
Continuous variables were compared using the ANOVA test. Categorical variables were compared using Pearson's χ2 test or Fisher's exact test, based on expected values. PFS and OS were estimated using the Kaplan–Meier method and compared using the log-rank test. The results are presented as the hazard ratio (HR) and 95% confidence interval (CI) according to Cox regression analyses. Propensity score matching (PSM) was performed to balance the clinical characteristics of the different treatment groups. In the current study, the groups of patients who switched EGFR-TKIs and patients without intolerable AEs served as the dependent variables, and the covariates included age, gender, PS, tumor morphology, smoking history, EGFR mutation subtypes, and liver and brain metastasis. Paired patients within the two groups with equivalent propensity scores were selected in a 1:4 manner.
IBM SPSS Statistics for Windows (Version 23.0) was used to perform all statistical analyses, and p < 0.05 was considered significant. Survival curves were plotted by SPSS. PSM was analyzed using the R package MatchIt.
Institutional review board statement
The study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (201901395B0).
RESULTS
The patterns of intolerable AEs
Among 2190 patients undergoing EGFR-TKIs, 114 (5.2%) patients experienced intolerable AEs that required discontinuation of EGFR-TKIs. In terms of different EGFR-TKIs, 25 (2.4%), 38 (7.7%), and 51 (8.0%) patients receiving gefitinib, erlotinib, and afatinib experienced intolerable AEs, respectively. After discontinuation of EGFR-TKIs, most patients (71.1%) received alternative EGFR-TKIs, 6.1% received chemotherapy without EGFR-TKIs, and 22.8% received no further anticancer treatment (Figure 1, Table S1).
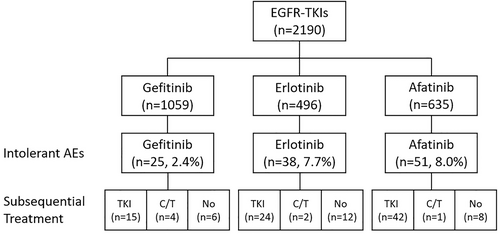
The types of intolerable AEs for patients receiving EGFR-TKIs differed according to the EGFR-TKI received. For the patients receiving gefitinib, the most common intolerable AEs were abnormal liver enzymes (LFT) (32.0%) followed by interstitial lung disease (ILD) (24.0%) and skin/paronychia/mucositis (16.0%). In contrast, skin/paronychia/mucositis (47.4%) was the most common intolerable AE, followed by diarrhea (18.4%), ILD (15.8%) and abnormal LFT (13.2%) for erlotinib. Diarrhea (43.1%) and skin/paronychia/mucositis (41.2%) were the most common AEs for afatinib (Table 1).
| Adverse event | Gefitinib (n = 25) | Erlotinib (n = 38) | Afatinib (n = 51) |
|---|---|---|---|
| Skin/paronychia/mucositis | 4 (16.0) | 18 (47.4) | 21 (41.2) |
| Skin | 3 | 18 | 14 |
| Paronychia | 1 | 0 | 3 |
| Mucositis | 0 | 0 | 4 |
| ILD | 6 (24.0) | 6 (15.8) | 2 (3.9) |
| LFT including hyperbilirubinemia | 8 (32.0) | 5 (13.2) | 1 (2.0) |
| GI other than diarrhea | 2 (8.0) | 0 | 2 (3.9) |
| Diarrhea | 1 (4.0) | 7 (18.4) | 22 (43.1) |
| Others | 4 (16.0) | 2 (5.3) | 3 (5.9) |
- Abbreviations: AEs, adverse events; EGFR-TKIs, epidermal growth factor receptor-tyrosine kinase; ILD, interstitial lung disease; LFT, liver function test; GI, gastrointestinal.
The clinical factors associated with intolerable AEs
To identify the factors that may predict intolerable AEs, the clinical factors including age, sex, PS, BW, BMI, and BSA were analyzed for the patients treated with different EGFR-TKIs. No clinical factors were significantly associated with intolerable AEs among the patients treated with gefitinib and erlotinib. Among the patients receiving afatinib, the patients with age >65, female gender, low BW, and low BSA were associated with higher rates of intolerable AEs (Table 2). In multivariate analysis, age >65 (odds ratio, [OR], 3.94; 95% CI, 2.02–7.68; p < 0.0001), female (OR, 2.35; 95% CI, 1.22–4.50; p = 0.010), BW (OR, 0.96; 95% CI, 0.93–0.99; p = 0.008), or BSA (OR, 0.07; 95% CI, 0.01–0.45; p = 0.005) were significantly associated with occurrence of intolerable AEs for the patients treated with afatinib. Of note, PS was not statistically significantly associated with intolerable AEs, but the poor PS patients had numerically higher rates of intolerable AEs (Table 2, Table S2).
| Gefitinib | Erlotinib | Afatinib | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (n = 25) | No (n = 1034) | p-value | Yes (n = 38) | No (n = 458) | p-value | Yes (n = 51) | No (n = 584) | p-value | |
| Age | 0.698 | 0.525 | <0.0001 | ||||||
| ≤65 | 9 (2.1) | 412 (97.9) | 14 (6.8) | 193 (93.2) | 12 (3.6) | 320 (96.4) | |||
| >65 | 16 (2.5) | 622 (97.5) | 24 (8.3) | 265 (91.7) | 39 (12.9) | 264 (87.1) | |||
| Sex | 0.966 | 0.104 | 0.008 | ||||||
| Male | 9 (2.4) | 368 (97.6) | 12 (5.5) | 207 (94.5) | 13 (4.8) | 260 (95.2) | |||
| Female | 16 (2.3) | 666 (97.7) | 26 (9.4) | 251 (90.6) | 38 (10.5) | 324 (89.5) | |||
| PS | 0.130 | 0.406 | 0.175 | ||||||
| 0 | 7 (4.3) | 154 (95.7) | 6 (9.4) | 58 (90.6) | 6 (4.4) | 129 (95.6) | |||
| 1 | 11 (1.7) | 631 (98.3) | 20 (6.4) | 291 (93.6) | 34 (8.6) | 363 (91.4) | |||
| More than 2 | 7 (2.7) | 249 (97.3) | 12 (9.9) | 109 (90.1) | 11 (10.7) | 92 (89.3) | |||
| BW, median (IQR) | 55 (18) | 56 (14) | 0.391 | 60 (15) | 59 (14) | 0.975 | 54 (18) | 59 (16) | 0.007 |
| BMI, median (IQR) | 23.6 (5.2) | 23.0 (4.7) | 0.395 | 24.5 (4.3) | 23.2 (5.0) | 0.271 | 22.6 (5.9) | 23.0 (4.4) | 0.182 |
| BSA, median (IQR) | 1.6 (0.3) | 1.6 (0.2) | 0.607 | 1.6 (0.3) | 1.6 (0.2) | 0.641 | 1.5 (0.2) | 1.6 (0.3) | 0.004 |
- Abbreviations: AEs, adverse events; BW, body weight; IQR, interquartile range; BMI, body mass index; BSA, body surface area.
The time to EGFR-TKIs discontinuation
The median time to EGFR-TKIs discontinuation was 2.56 months (95% CI, 0.31–40.67 months). No significant difference was noted among different EGFR-TKIs (p = 0.936). Skin/paronychia/mucositis had the longest time to discontinuation (median 6.21 months), followed by abnormal LFT (median, 4.48 months), and GI AEs other than diarrhea had the shortest longest time to discontinuation (median, 0.69 months) (Figure 2). [Correction added on 20 January 2023, after first online publication: the preceding sentence has been revised. The original sentence read: Skin/paronychia/mucositis had the longest time to discontinuation (median 6.21 months), followed by abnormal LFT (median, 4.48 months), and ILD had the shortest longest time to discontinuation (median, 0.69 months) (Figure 2).]

Survivals based on EGFR-TKIs and types of AEs
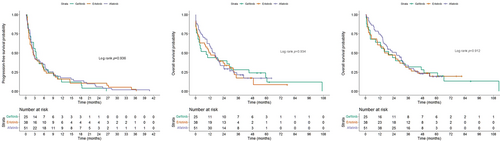
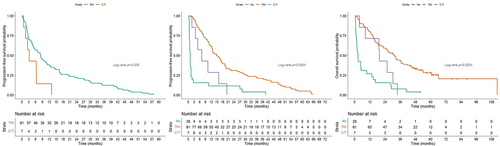
Further analysis was performed to understand the impact of EGFR-TKIs and type of AEs on survivals of the patients experiencing intolerable AEs. Regarding different EGFR-TKIs, the time to discontinuation (Figure 3(a)), OS from discontinuation (Figure 3(b)), and OS (Figure 3(c)) did not show a significant difference. In contrast, different types of AEs influenced the time to discontinuation (Figure 4(a)), OS from discontinuation (Figure 4(b), Table S3), and OS (Figure 4(c), Table S4). The patients with skin/paronychia/mucositis and abnormal LFT had better survivals than the patients with other intolerable AEs such as ILD and GI AEs other than diarrhea.
Survivals based on subsequent treatment
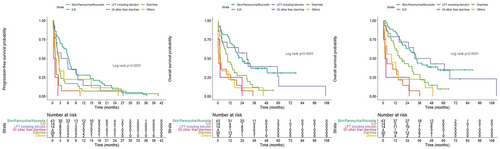
Further investigation of survivals based on subsequent treatment was performed because not all patients would switch to alternative EGFR-TKIs, possibly because of fear of AE recurrence or rapid progression of the underlying disease. The patients receiving subsequent EGFR-TKI had significantly better PFS than those who received subsequent chemotherapy (median PFS, 8.0 vs. 3.1 months; HR, 2.41; 95% CI, 1.09–5.33; p = 0.030) (Figure 5a). In terms of total PFS from frontline EGFR-TKIs to the first progression, the patients receiving subsequent EGFR-TKIs (median, 14.9 months; 95% CI, 11.0–18.8 months) experienced the best total PFS than those receiving chemotherapy (median, 7.0 months, 95% CI, 1.7–12.3 months) and those without subsequent treatment (median, 0.9 months; 95% CI, 0.6–1.2 months) (Figure 5(b)). The patients receiving subsequent EGFR-TKIs (median, 31.3 months; 95% CI, 23.9–38.7 months) had the best OS compared to those receiving chemotherapy (median, 19.4 months; 95% CI, 18.5–20.3 months) and those without subsequent treatment (median, 2.4 months; 95% CI, 1.3–3.5 months) (Figure 5(c)).
Comparison between the patients with and without intolerable AEs
As the patients receiving subsequent EGFR-TKIs had favorable survivals, further comparison between these patients and those without intolerable AEs was performed. Overall, the patients receiving subsequent EGFR-TKIs had better total PFS (median, 14.9 vs. 11.3 months; p = 0.013) and OS (median, 31.3 vs. 20.1 months; p = 0.001) than the patients without discontinuation because of AEs (Figure 6(a),(b)).
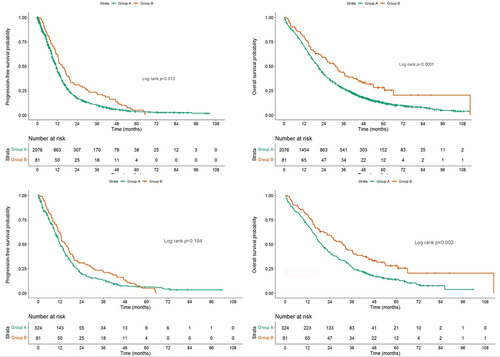
To avoid imbalance among clinicopathological features (age, gender, mutation status, smoking history, stage, frontline EGFR-TKIs, brain metastasis, and liver metastasis) that may serve as confounding factors influencing survival, PSM was performed using a 1:4 ratio. All the factors became balanced after PSM (Table 3). The patients with subsequent EGFR-TKIs had a trend of better PFS (14.9 vs. 12.0 months, p = 0.194) and significantly better OS (31.3 vs. 19.5 months, p = 0.002) than patients without intolerable AEs (Figure 6(c),(d)). PSM was also performed with ratios of 1:1, 1:2, 1:3, and 1:4 (Table S5), and the patients who had intolerable AEs and received a different EGFR-TKIs after the AE had a trend of better PFS and significantly better OS than those who did not have intolerable AEs (Figure S1).
| Discontinuation | Primary cohort | PSM 1:4 | ||||
|---|---|---|---|---|---|---|
| Yes | No | p-value | Yes | No | p-value | |
| N = 81 | N = 2076 | N = 81 | N = 324 | |||
| Age (years), median (IQR) | 73 (20) | 67 (18) | 0.006 | 73 (20) | 69 (18) | 0.477 |
| ≦65 | 27 (33.3) | 925 (44.6) | 0.046 | 27 (33.3) | 118 (36.4) | 0.604 |
| >65 | 54 (66.7) | 1151 (55.4) | 54 (66.7) | 206 (63.6) | ||
| Gender | 0.001 | 0.567 | ||||
| Male | 17 (21.0) | 835 (40.2) | 17 (21.0) | 59 (18.2) | ||
| Female | 64 (79.0) | 1241 (59.8) | 64 (79.0) | 265 (81.8) | ||
| Performance score | 0.928 | 0.840 | ||||
| 0 | 12 (14.8) | 341 (16.4) | 12 (14.8) | 45 (13.9) | ||
| 1 | 51 (63.0) | 1285 (61.9) | 51 (63.0) | 215 (66.4) | ||
| 2/3/4 | 18 (22.2) | 450 (21.7) | 18 (22.2) | 64 (19.8) | ||
| Morphology | 0.169 | 0.778 | ||||
| Adenocarcinoma | 78 (96.3) | 2041 (98.3) | 78 (96.3) | 314 (96.9) | ||
| Non-adenocarcinoma | 3 (3.7) | 35 (1.7) | 3 (3.7) | 10 (3.1) | ||
| Mutation | 0.010 | 0.535 | ||||
| 19del | 27 (33.3) | 993 (47.8) | 27 (33.3) | 120 (37.0) | ||
| L858R | 54 (66.7) | 1083 (52.2) | 54 (66.7) | 204 (63.0) | ||
| Stage | 0.513 | 0.766 | ||||
| IIIB | 4 (4.9) | 141 (6.8) | 4 (4.9) | 14 94.3) | ||
| IV | 77 (95.1) | 1935 (93.2) | 77 (95.1) | 310 (95.7) | ||
| Drug | <0.0001 | 0.701 | ||||
| Afatinib | 42 (51.9) | 584 (28.1) | 42 (51.9) | 157 (48.5) | ||
| Erlotinib | 24 (29.6) | 458 (22.1) | 24 (29.6) | 112 (34.6) | ||
| Gefitinib | 15 (18.5) | 1034 (49.8) | 15 (18.5) | 55 (17.0) | ||
| Liver | 0.113 | 0.854 | ||||
| Yes | 6 (7.4) | 280 (13.5) | 6 (7.4) | 26 (8.0) | ||
| No | 75 (92.6) | 1796 (86.5) | 75 (92.6) | 298 (92.0) | ||
| Brain | 0.045 | 0.919 | ||||
| Yes | 32 (39.5) | 605 (29.1) | 32 (39.5) | 130 (40.1) | ||
| No | 49 (60.5) | 1471 (70.9) | 49 (60.5) | 194 (59.9) | ||
- Abbreviation: PSM, propensity score matching; IQR, interquartile range;
DISCUSSION
In the current study, 5.2% of patients experience intolerable AEs requiring discontinuation of EGFR-TKIs. The median time to EGFR-TKIs discontinuation was 2.56 months and varied for different types of AE. Sex, gender, body weight, and body surface area were significantly associated with the occurrence of intolerable AEs for the patients treated with afatinib, but not gefitinib nor erlotinib. The patients experiencing skin/paronychia/mucositis and abnormal liver function test had favorable survivals. The patients who received a different EGFR-TKI after the AE experienced the best PFS, total PFS (from frontline line EGFR-TKIs), and OS than those who subsequently received chemotherapy or no treatment. The patients receiving a different EGFR-TKI after the AE had better total PFS and OS than the patients who did not discontinue because of an AE. The favorable OS was validated by PSM.
In the current study, 114 (5.2%) of 2190 patients experienced intolerable AEs that required discontinuation of EGFR-TKIs. The discontinuation rates for each EGFR-TKI and the reported AEs leading to discontinuation in this report were similar to those reported in previous studies. The discontinuation rates of EGFR-TKIs varied among previous studies. The discontinuation rates for afatinib and gefitinib were 6% in the LUX-Lung 7 study.3 The discontinuation rates were 13% and 18% for osimertinib and standard EGFR-TKIs (gefitinib/erlotinib), respectively, in the FLAURA study.5 The data should be interpreted carefully because direct comparison may not be feasible because of distinct study designs and different populations. Interestingly, the types of AEs differed according to the different EGFR-TKIs in the current cohort, which is expected, but few studies reported this finding.
In the current study, skin AEs included skin rashes, erythema, folliculitis, skin eruptions, and other cutaneous manifestations, which are typical among patients treated with EGFR-TKIs. Unlike paronychia and mucositis, these AEs are typically considered tolerable, with limited cases requiring discontinuation because of the occurrence of these AEs (Table 1). Therefore, this study collectively categorized all skin-related AEs into a single category, named “skin/paronychia/mucositis”.
No specific clinical features were associated with patients treated with gefitinib and erlotinib, implying that both gefitinib and erlotinib had similar tolerability across whole subpopulations. In contrast, the patients with older age, female gender, and low BW and BSA experienced a higher rate of discontinuation from afatinib than the products, implying that the tolerability of afatinib was not universal. The use of EGFR-TKI in such patients requires much more attention to AEs and AEs can be managed not just by treatment, but also through dose reduction. Plasma concentrations of EGFR-TKI may be associated with the occurrence of AEs, based on findings from previous prospective studies7 and may be used to predict AEs. However, because of the retrospective nature of this study, plasma EGFR-TKI concentrations were not available, preventing the evaluation of associations between plasma EGFR-TKI concentrations and AE occurrence.
The median time to discontinuation was 2.56 months and varied for different types of AE. In contrast to ILD and GI AEs, which may lead to severe consequences and death, skin/paronychia/mucositis was generally tolerable even when patients experienced a high level of this AE. This can explain the different times to discontinuation for different intolerable AEs. Skin/paronychia/mucositis can be relieved and recovered from with short-term discontinuation (several days) of EGFR-TKIs, so these patients had favorable survivals over the patients with potentially lethal AEs such as ILD, which should be treated for weeks before beginning any subsequent treatment.
In the current study, the patients who received subsequent EGFR-TKIs experienced more favorable survivals than those who received subsequent chemotherapy or no subsequent treatment. The patients receiving subsequent EGFR-TKIs had better total PFS and OS than the patients without discontinuation because of AEs. PSM validated the favorable OS. In a Japanese cohort, five patients experienced severe intolerable AEs and switched to alternative EGFR-TKIs. The median PFS for alternative EGFR-TKIs was 11.7 months, which was better than the patients switching EGFR-TKIs because of progression.13 Another Japanese cohort showed 17 patients who switched EGFR-TKIs because of intolerable AEs, and the median PFS for the second EGFR-TKI treatment was 15.2 months.14 Both studies showed switching EGFR-TKIs was an efficacious strategy for the patients experiencing intolerable AEs, but the case number is limited. The 114 patients enrolled in the current study provide better real-world experience for patients with intolerable AEs. Furthermore, previous studies did not identify a control group of patients with intolerable AEs, which limited their novelty. In the current study, we found that the patients switching to EGFR-TKIs had better survivals than those switching to chemotherapy or best supportive care. Interestingly, the patients who switched also had better results than patients who did not experience intolerable AEs. This may be because of lower plasma EGFR-TKI concentrations in patients without intolerable AEs compared with patients who experienced intolerable AEs, based on the findings from a previous report.7 However, we did not have plasma EGFR-TKI concentrations measurements in this retrospective study.
CONCLUSIONS
In conclusion, NSCLC patients experiencing intolerable AEs during EGFR-TKI treatment should consider switching to an alternative EGFR-TKI. Age, gender, BW, and BSA may be the predictors for EGFR-TKI discontinuation. Switching to alternative EGFR-TKIs should be the optimal option with favorable survival outcomes for those who discontinue EGFR-TKIs because of toxicities.
ACKNOWLEDGMENT
This work was supported by Chang Gung Research Database. This work was supported by Linkou Chang-Gung Memorial Hospital (CMRPG3M0971 to C.-E.W.)
CONFLICT OF INTEREST
The authors declare no conflict of interest.