Response and safety of whole-brain radiotherapy plus temozolomide for patients with brain metastases of non-small-cell lung cancer: A meta-analysis
Funding information: Natural Science Foundation of Inner Mongolia, Grant/Award Numbers: 2015MS0859, 2019BS08007; Natural Science Foundation of Shenzhen University General Hospital, Grant/Award Number: SUGH2018QD023
Abstract
Objective
The aim of the present work was to investigate the response and safety of whole-brain radiotherapy (WBRT) plus temozolomide (TMZ) for patients with brain metastases of non-small-cell lung cancer (NSCLC).
Methods
The electronic databases of Pubmed, EMbase, Cochrane, Wangfang, china national knowledge infrastructure (CNKI), and Google scholar were systematically searched to identify the prospective randomized trials relevant to WBRT plus TMZ for patients with brain metastases of NSCLC. The data associated with treatment response and toxicity were extracted from original included studies. The relative risk (RR) for treatment response and toxicity between WBRT+TMZ and WBRT alone was pooled by fixed or random effect model. Publication bias was investigated by Begg's funnel plot and Egger's line regression test.
Results
Twenty-five clinical trials fulfilled the inclusion criteria and were included in the meta-analysis. The pooled results showed WBRT+TMZ can significant improve the objective response rate (ORR) compared with WBRT alone (RR = 1.43, 95% confidence interval [CI] 1.32–1.55, p < 0.05) under a fixed effect model. WBRT+TMZ significantly increased the III–IV hematological toxicity compared to WBRT alone (RR = 1.66, 95% CI 1.12–2.54, p < 0.05) in the fixed effect model. Grade III–IV gastrointestinal toxicity was increased in WBRT+TMZ compared to WBRT alone (RR = 1.72, 95% CI 1.29–2.30, p < 0.05). Begg's funnel plot and Egger's line regression test indicated publication bias.
Conclusion
Based on the present work, WBRT+TMZ can improve the ORR for brain metastases of NSCLC, but the risk of treatment-associated grade III/IV hematological toxicity and gastrointestinal toxicity were also increased compared to WBRT alone.
INTRODUCTION
Brain metastases are the most common intracranial malignant tumors.1, 2 Most of their primary lesions come from the lung.3 The brain metastasis rate of patients with non-small-cell lung cancer (NSCLC) is as high as 44%.4 NSCLC is the main cause of cancer death worldwide, and more than 20% of patients have brain metastasis during treatment.5 Brain involvement is an independent adverse prognostic factor with a median natural survival time of 1–2 months. The median survival time is only 4–12 months even with effective treatment.6 Therefore, brain metastasis is the main cause of treatment failure in patients with lung cancer. After receiving whole-brain radiation therapy (WBRT) as the standard treatment mode, the overall survival of NSCLC patients with brain metastasis can be prolonged by 3–6 months.7, 8 However, patients with brain metastasis have heavy tumor load, and radiotherapy alone has limited survival benefit, therefore the effect of WBRT alone is unsatisfied, and the recurrence rate in 6 months is as high as 70%.9
Platinum combined with third-generation cytotoxic chemotherapy drugs can bring survival benefits to brain metastasis of NSCLC patients.10 However, the existence of the blood–brain barrier limits the therapeutic effect and the treatment toxicity risk is significant increased.
Temozolomide (TMZ) is an imidazole tetrazine alkylating agent with antitumor activity. In vivo, TMZ is rapidly transformed into the active product 3-methyl-[triazine-1-]imidazole-4-formamide (MTIC). The therapeutic benefit of TMZ depends on its ability to alkylate/methylate DNA, which most often occurs at the N-7 or O-6 positions of guanine residues.11 Studies have indicated that TMZ combined with WBRT had a higher response rate and longer progression-free survival time compared with WBRT alone in the treatment of patients with brain metastasis of NSCLC.12 In the present work, we compared the objective response rate (ORR) and treatment-associated toxicity between WBRT+TMZ and WBRT only through pooling open published data, which may provide more evidence for brain metastases of NSCLC.
MATERIAL AND METHODS
Clinical trials electronic searching
The publication electronic searching was performed by two authors (J.H. and S.D.). The electronic databases of Pubmed, EMbase, Cochrane, Wangfang, china national knowledge infrastructure, and Google scholar were systematically searched to identify the prospective randomized trials relevant to WBRT plus TMZ for patients with brain metastases of NSCLC. The following free-text words were applied to initially identify studies: carcinoma, non-small-cell lung; carcinoma, non-small-cell lung cancer; NSCLC; non-small-cell lung carcinoma; brain metastasis; brain metastases; temozolomide; temodar; TMZ; temozolamide; whole-brain radiotherapy; WBRT.
Studies inclusion and exclusion criteria
- Prospective clinical studies relevant to WBRT+TMZ versus WBRT alone for patients with brain metastases of NSCLC.
- Study published in English or Chinese.
- Data for ORR and treatment toxicity can be extracted from the original trial.
- Patients were diagnosed with brain metastases of NSCLC.
- Response evaluated by CT or MRI of the brain.
- National cancer institute-common terminology criteria for adverse events applied for toxicity evaluation.
- Retrospective clinical study or case report.
- Brain metastases of other malignant tumor other than NSCLC.
- ORR or treatment toxicity can't be extracted or calculated from the original study.
Data extraction
The information and data for each included study were extracted by two authors (J.H. and M.Q.) and cross-checked. The following general information was extracted:
(i) First and corresponding authors.
(ii) The date of the trial was published.
(iii) The country where the trial was performed.
(iv) Mean, median or range of the ages of included cases.
(v) Gender of the cases in each included study.
(vi) Karnofsky performance scale (KPS) or eastern cooperative oncology group (ECOG) score.
- ORR for the WBRT+TMZ and WBRT groups in each study.
- Number of grade III–IV hematological toxicities, such as neutropenia, thrombocytopenias and anemia, in the WBRT+TMZ and WBRT only groups in each study.
- Number of grade III–IV gastrointestinal toxicities in the WBRT+TMZ and WBRT only groups in each study.
Study quality assessment
The methodological qualities of each included study were evaluated by two authors (W.T. and S.D.) for aspects of allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other bias factors that generally represent the quality of the randomized controlled trial.
Publication bias assessment
The Begg's funnel plot and Egger's line regression test were applied for publication bias analysis. If the funnel plot was left–right symmetrical and Eggerʼ test p > 0.05, it was considered to be no publication bias. Otherwise, the meta-analysis was assumed to have publication bias.
Statistical analysis
Review Manager (RevMan 5.0, Cochrane Collaboration) was applied for trial quality evaluation. Stata12.0 (Stata Corporation, http://www.stata.com) was used for data combination. Statistical heterogeneity between studies was evaluated by the chi-squared test. If p < 0.05, data were pooled by a random effect model and otherwise by a fixed effect model. Publication bias was evaluated by Begg's funnel plot and Egger's line regression test. Two tails p < 0.05 was considered to be statistical significance.
RESULTS
Studies searching results
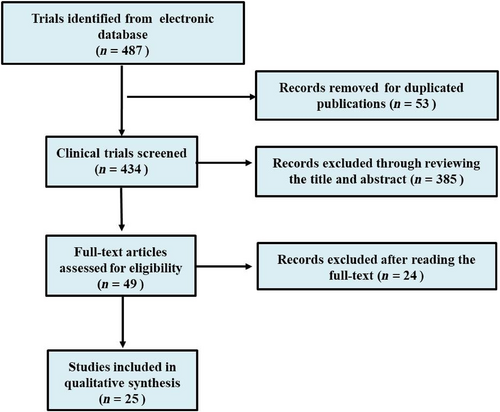
Four hundred eighty-seven clinical studies relevant to WBRT plus TMZ for patients with brain metastases of NSCLC were initially identified after searching the electronic databases. Twenty-five clinical trials fulfilled the inclusion criteria and were included in the meta-analysis. The study searching and inclusion procedure is shown in Figure 1. The main characteristics of the 25 studies included are shown in Table 1.
| Trials | Year | Country | WBRT+TMZ | WBRT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | Age (years) | Gender (M/F) | KPS | Sample size | Age (years) | Gender (M/F) | KPS | |||
| Antondou13 | 2003 | Greece | 52 | NA | NA | NA | 51 | NA | NA | NA |
| Chua14 | 2010 | China | 47 | 59 (38–78) | 30/17 | NA | 48 | 62 (43–79) | 32/16 | NA |
| Hassler15 | 2013 | Austria | 22 | 69 (36–85) | NA | 80 (7–100) | 13 | 64 (54–78) | NA | 80 (70–100) |
| Xie16 | 2007 | China | 25 | NA | NA | NA | 25 | NA | NA | NA |
| Zhou17 | 2011 | China | 20 | 61 | 14/6 | NA | 22 | 55 | 15/7 | NA |
| Shi18 | 2014 | China | 43 | 55 (38072) | 25/18 | NA | 41 | 56 (38–73) | 24/17 | NA |
| Yang19 | 2015 | China | 23 | NA | NA | NA | 22 | NA | NA | NA |
| Gu20 | 2015 | China | 52 | 59.5 ± 5.7 | 28/24 | 68.3 ± 5.2 | 50 | 58.2 ± 5.2 | 23/27 | 67.0 ± 4.5 |
| Sun21 | 2016 | China | 30 | 59.0 ± 6.9 | 17/13 | 0.97 ± 0.76 (ECOG) | 30 | 58.5 ± 7.6 | 16/14 | 0.90 ± 0.66 (ECOG) |
| Zhi22 | 2016 | China | 44 | 37–75 | NA | NA | 44 | 35–37 | NA | NA |
| Liu23 | 2016 | China | 21 | NA | NA | >70 | 16 | NA | NA | >70 |
| Mu24 | 2016 | China | 29 | NA | NA | NA | 29 | NA | NA | NA |
| Xu25 | 2016 | China | 40 | NA | NA | NA | 40 | NA | NA | NA |
| Zhao26 | 2016 | China | 30 | 38–69 | 16/14 | NA | 30 | 36–68 | 17/13 | NA |
| Wang27 | 2017 | China | 37 | NA | 18/19 | ≧70 | 41 | NA | 22/19 | ≧70 |
| Teng28 | 2017 | China | 26 | 55.6 ± 7.8 | 16/10 | ECOG(0–2) | 25 | 56.8 ± 9.8 | 14/11 | ECOG (0–2) |
| Sperduto29 | 2013 | U.S | 40 | 63 | NA | NA | 44 | 64 | NA | NA |
| Li30 | 2015 | China | 18 | 54.5 (39–70) | 10/8 | NA | 18 | 53.5 (37–72) | 11/7 | NA |
| Jiang31 | 2015 | China | 22 | 41–81 | NA | NA | 22 | 41–81 | NA | NA |
| Deng12 | 2017 | China | 129 | NA | 60/69 | ECOG (0–3) | 109 | 42/67 | NA | ECOG (0–3) |
| Wu32 | 2013 | China | 20 | 10/10 | 40–47 | NA | 20 | 10/10 | 40–47 | NA |
| Hasyatti33 | 2018 | China | 25 | NA | NA | NA | 25 | NA | NA | NA |
| Wan34 | 2018 | China | 28 | 54.6 ± 6.3 | 16/12 | ≧70 | 28 | 52.0 ± 6.3 | 15/13 | ≧70 |
| Lu35 | 2019 | China | 40 | 53.57 ± 12.83 | 30/10 | NA | 40 | 23/17 | 54.27 ± 11.90 | NA |
| Wang36 | 2020 | China | 25 | NA | NA | NA | 25 | NA | NA | NA |
- Abbreviations: ECOG, eastern cooperative oncology group; KPS, karnofsky performance scale; NA, not available.
Quality of the included studies
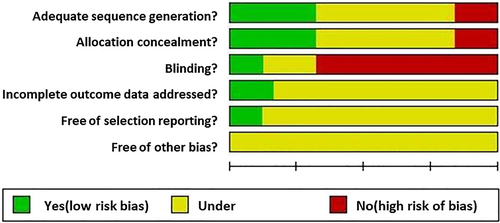
The methodical qualities of the 25 included studies were supposed to be moderate risk of bias. Most of the studies did not report the blinding status. The detailed quality assessment results are shown in Figure 2.
Statistical heterogeneity evaluation
The chi-squared test showed no significant statistical heterogeneity across the 25 included trials for the effect size of the ORR (I2 = 0.00%, p = 0.989), hematological toxicity (I2 = 6.80%, p = 0.379), and gastrointestinal toxicity (I2 = 37.2%, p = 0.121), therefore the data were pooled by fixed effect model.
Objective response
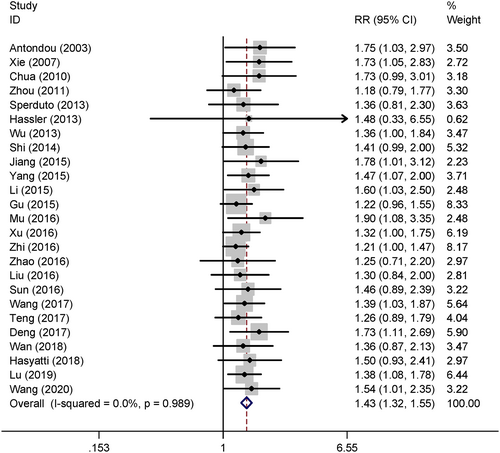
Twenty-five studies reported the treatment response and the pooled results showed WBRT+TMZ can significantly improve the ORR compared WBRT alone (relative risk [RR] = 1.43, 95% confidence interval [CI] 1.32–1.55, p < 0.05) in a fixed effect model (Figure 3).
Treatment-related toxicity
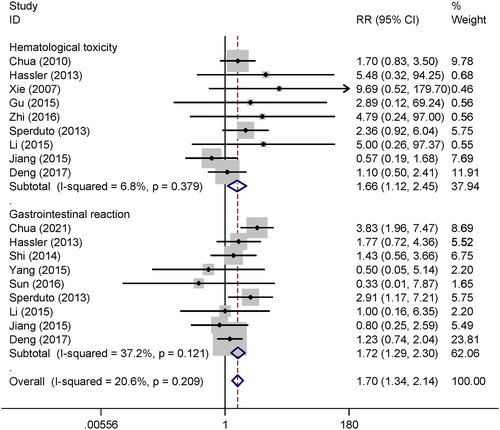
Nine studies reported grade III–IV treatment-related hematological toxicity. The pooled results show that WBRT+TMZ significantly increased the grade III–IV hematological toxicity compared to WBRT alone (RR = 1.66, 95% CI 1.12–2.54, p < 0.05) under the fixed effect model. Nine trials compared the gastrointestinal toxicity between WBRT+TMZ and WBRT alone and the combined results indicate that grade III–IV gastrointestinal toxicity is significant increased in WBRT+TMZ compared to WBRT alone (RR = 1.72, 95% CI 1.29–2.30, p < 0.05) by the fixed effect model (Figure 4).
Publication bias
The Begg's funnel plots for ORR and toxicity were left–right asymmetrical, which indicates significant publication bias (Figure 5). Egger's line regression test also showed publication bias (p < 0.05).
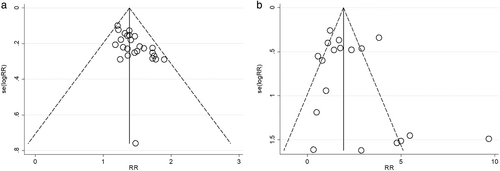
DISCUSSION
After comprehensive systematic electronic searching, 25 clinical trials were included and quantitative analysis was performed. The pooled results indicated that the ORR in the WBRT+TMZ group was 1.43 times higher than that of the WBRT only group with statistical difference. However, WBRT+TMZ also significant increased the 66% grade III/IV hematological toxicity and the 72% grade III–IV gastrointestinal toxicity risk compared to WBRT alone. Therefore, the short-term ORR was improved by adding TMZ in the treatment of brain metastases from NSCLC, but the treatment-associated toxicity risk was also increased.
The present meta-analysis combined 25 open published clinical trials. Most of the work was done in China on the Chinese population. Only three studies were performed in United States, Greece, and Australia, therefore patient selection bias was ineluctable. In addition, the general methodical quality of the 25 studies was moderate. Most of the studies did not report the “blinding” status, which was at high risk of bias.
TMZ is a conventional chemotherapy drug applied in the treatment of intracranial tumors. As a new imidazole tetrazine alkylating agent, it has the characteristics of small molecular weight, wide antitumor spectrum, lipophilicity, and ability to pass through the blood–brain barrier.37 The therapeutic benefit of TMZ depends on its ability to alkylate/methylate DNA, which most often occurs at the N-7 or O-6 positions of guanine residues. In some tumors, epigenetic silencing of the O-6-methylguanine-DNA methyltransferase gene prevents the synthesis of this enzyme, and as a consequence such tumors are more sensitive to killing by TMZ. After oral administration, TMZ concentration in brain tissue can reach 30–40% of that in blood, and the bioavailability is close to 100%.38 Moreover, it can concentrate on tumor sites in vivo, with strong efficacy, certain selectivity, and fewer side effects. It has therefore has become the first-line chemotherapy drug for the clinical treatment of malignant brain tumors such as glioblastoma multiforme and anaplastic astrocytoma. Due to the satisfied anti-intracranial tumor effects of TMZ, several studies have investigated its effects on brain metastases diseases combined with WBRT. Chua14 and his colleges performed a randomized, open-label phase II clinical trial relevant to WBRT plus concomitant TMZ for the treatment of brain metastases from NSCLC. The authors recruited 95 brain metastases cases of NSCLC (47 in WBRT+TMZ, 49 in WBRT only) and found that the overall survival and central nervous system progression survival were not statistically different between WBRT+TMZ and WBRT only groups. The benefit of adding TMZ to WBRT was not confirmed. However, Deng12 included a total of 238 NSCLC patients with brain metastases who received WBRT+TMZ or WBRT, respectively. The results showed adding TMZ to WBRT could improve the intracranial ORR and median progression-free survival compared with WBRT alone. However, side effects were also increased by adding TMZ, but the difference was not statistically significant and toxicities were well tolerated. Chua and Deng got inconclusive results on the ORR and survival for WBRT+TMZ versus WBRT alone for brain metastases of NSCLC.
Our meta-analysis combined 25 open published studies and indicated that the short-term ORR was improved by adding TMZ in the treatment of brain metastases from NSCLC, but the treatment-associated toxicity risk was also increased, which is in accordance with Deng's conclusion.
In conclusion, based on the present work, WBRT+TMZ can improve the ORR for brain metastases of NSCLC, but the risk of treatment-associated grade III/IV hematological toxicity and gastrointestinal toxicity was also increased compared to WBRT only. However, due to the aforementioned limitation of patient selection bias, the moderate methodical quality of included studies, and publication bias, more well-designed multicenter prospective randomized clinical controlled studies are urgently need to further validate the findings.
ACKNOWLEDGMENT
This work was supported by Natural Science Foundation of Inner Mongolia Grant numbers 2015MS0859, 2019BS08007 and Natural Science Foundation of Shenzhen University General Hospital Grant number SUGH2018QD023.




