Learning curve of robotic portal lobectomy for pulmonary neoplasms: A prospective observational study
The main data of this paper were presented in a poster presentation at the 2019 AATS International Thoracic Surgical Oncology Summit on 27–28 September 2019 at New York, USA (Abstract Control Number: 48, Poster Number: 10).
Funding information: National Natural Science Foundation of China, Grant/Award Number: 82072572; Natural Science Foundation of Guangdong Province, Grant/Award Numbers: 2018A030313410, 2020A151501311; Sun Yat-sen University Clinical Research 5010 Program, Grant/Award Number: 2019012
Abstract
Background
We aim to assess the learning curve of robotic portal lobectomy with four arms (RPL-4) in patients with pulmonary neoplasms using prospectively collected data.
Methods
Data from 100 consecutive cases with lung neoplasms undergoing RPL-4 were prospectively accumulated into a database between June 2018 and August 2019. The Da Vinci Si system was used to perform RPL-4. Regression curves of cumulative sum analysis (CUSUM) and risk-adjusted CUSUM (RA-CUSUM) were fit to identify different phases of the learning curve. Clinical indicators and patient characteristics were compared between different phases.
Results
The mean operative time, console time, and docking time for the entire cohort were 130.6 ± 53.8, 95.5 ± 52.3, and 6.4 ± 3.0 min, respectively. Based on CUSUM analysis of console time, the surgical experience can be divided into three different phases: 1–10 cases (learning phase), 11–51 cases (plateau phase), and >51 cases (mastery phase). RA-CUSUM analysis revealed that experience based on 56 cases was required to truly master this technique. Total operative time (p < 0.001), console time (p < 0.001), and docking time (p = 0.026) were reduced as experience increased. However, other indicators were not significantly different among these three phases.
Conclusions
The RPL-4 learning curve can be divided into three phases. Ten cases were required to pass the learning curve, but the mastery of RPL-4 for satisfactory surgical outcomes requires experience with at least 56 cases.
INTRODUCTION
In the early 2000s, robotic-assisted thoracoscopic surgery (RATS) was first applied for lung resection.1 Previous studies demonstrated the safety and feasibility of using RATS in non-small cell lung cancer (NSCLC),2-6 and the long-term outcomes of RATS are not inferior to video-assisted thoracic surgery (VATS) and thoracotomy procedures.7-9 A frequent concern regarding any new technology is the number of procedures required to gain confidence by a new adopter, the so-called learning curve.
Learning curves may vary among different studies due to the use of different generations of robot devices and surgical approaches as well as individual experiences in minimally invasive surgeries.2, 10-14 It is therefore necessary to define learning curves based on procedure experience using prospectively collected data.
Recently, a new nomenclature was proposed to describe a completely portal robotic lobectomy with four arms, namely, RPL-4.15, 16 In this study, we included 100 consecutive RPL-4s for pulmonary neoplasms performed by a single surgical team in a single institution using the Da Vinci Si system (Intuitive Surgical Inc.) to investigate the learning curve of this procedure.
METHODS
Patient selection
This prospective observational analysis was designed to accumulate peri/postoperative data from 100 consecutive patients with pulmonary neoplasms undergoing RPL-4 between June 2018 and August 2019. The Institutional Review Board of Sun Yat-sen University Cancer Center approved this study (No. RDDA2019001108) on 7 July 2019. Informed consent for this study was deemed not required, as it did not involve any therapy beyond those routinely performed and administered during standard patient care before, during and after surgery. There was no additional risk to the involved patients.
The inclusion criteria for this study were as follows: (i) age from 18 to 80; (ii) diagnosed as primary lung neoplasms indicated for lobectomy; (iii) clinical stage I–II and resectable IIIa; and (iv) cardiopulmonary function test results suggested that the patient could tolerate lobectomy.
The exclusion criteria for this study were as follows: (i) surgeries other than lobectomy: wedge resection, segmentectomy, bilobectomy, and pneumonectomy; (ii) diagnosed as ground glass opacity; and (iii) history of thoracotomy at the same side.
Data collection
A comprehensive case report form was previously designed for each included case. The general clinical characteristics such as gender, age, tumor location, anatomical type, tumor size, smoking history, comorbidity, and clinical stage were recorded before the operation. The cost of time for each step of the operation, such as the console time, docking time, the time for dissection of individual station of lymph node (LN), the time for taking individual hilar structure, and the time for incision closure, were recorded simultaneously during surgery. In addition, the blood loss, perioperative complications, and the reason for conversion (if occurred) were also recorded during each operation. The pathological stage, number of mediastinal LNs harvested, number of total LNs harvested, chest tube duration, length of stay in hospital, and cost were recorded after operation. The 8th edition Lung Cancer Stage Classification of the American Joint Committee on Cancer17 was applied in this study. Peri/postoperative complications were recorded and classified according to the Clavien–Dindo Classification System.18
Outcome
The primary endpoints of this study were total operative time and console time of the first consecutive 100 cases, which was the basis for defining the learning curve of RPL-4. The secondary endpoints of this study were docking time, number of mediastinal LNs harvested, number of total LNs harvested, blood loss during surgery, chest tube duration, length of stay in hospital, and cost.
Operative team
One surgical team led by Dr. Hao-Xian Yang as the attending surgeon performed all of the 100 surgeries. Three surgical assistants participated in the operation procedures during the study period (Mu-Zi Yang, Jin-Chun Wu, and Gang Wang). All of the circulating nurses and scrub nurses were from the same robotic surgery team.
Operative technique
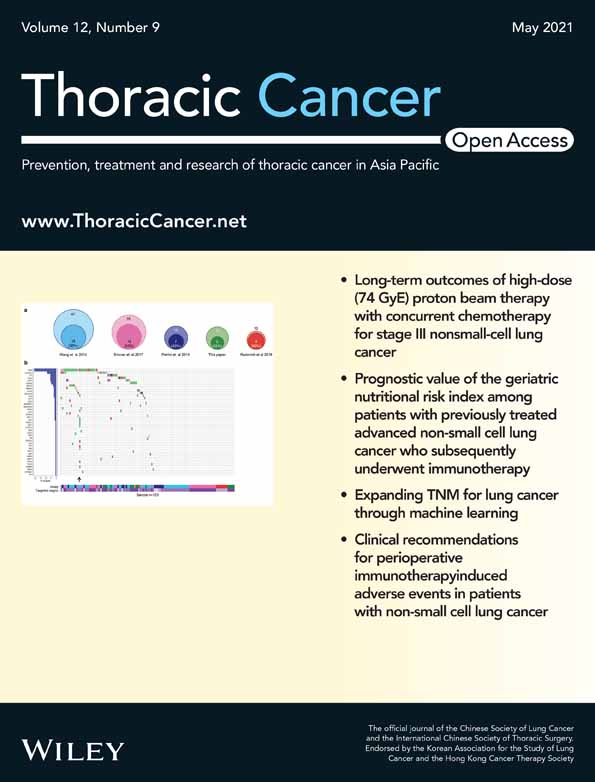
RPL-4 procedures were used in this cohort.15, 16 Before initiating the robotic operation, the attending surgeon (Dr. Hao-Xian Yang) observed robotic surgeries at the Memorial Sloan-Kettering Cancer Center,9 the University of Alabama at Birmingham, the General Hospital of Shenyang Northern Theater Command, and Shanghai Chest Hospital. The surgeon was certified with the first assistant and console surgeon in Hong Kong (2016) and Shanghai (2018), respectively. Double lumen tracheal tube insertion was universally applied for anesthesia. The four arms of the Da Vinci Si system were used for all cases. Three 8-mm ports were used for the robotic instruments, and one 12-mm port was used for the robotic camera combined with a 12-mm assistant port. The five portal incisions were only large enough for the size of their individual trocars. The assistant port was enlarged after undocking the robot to extract the specimen. Figure 1 shows the port location strategy of RPL-4 based on the specific lobe. Each adjacent port was made 8–10 cm apart to avoid obstruction between instruments and CO2 insufflation was performed at 5–7 mmHg. Once the trocars were all placed, the robot was docked over the head of the patient.
General statistical analysis
Statistical analysis was performed using IBM SPSS Statistics (version 20.0, IBM Corp.). A two-sided p < 0.05 was considered statistically significant. Descriptive analysis was presented as the mean ± standard deviation for continuous variables and frequency with percentages for categorical variables. Group differences were compared. Continuous variables were compared by Mann–Whitney U test or Kruskal–Wallis H test for continuous variables and by Pearson χ2 test or Fisher's exact test for categorical variables.
Cumulative sum analysis
In this study, the learning curve was analyzed by cumulative sum (CUSUM) analysis. This method has been proven to be effective in learning curve analyses, especially in the field of surgery.10, 13, 19, 20 In this study, the average total operative time was primarily calculated (cases with conversion to open surgery were excluded), and the CUSUM statistic of the first case was equal to the total operative time of the first case minus the average total operative time. CUSUM statistics of the second and subsequent cases were equal to the difference between total operative time and the average added CUSUM statistic of the previous case.10, 13, 19, 20 Because our hospital is a university hospital, we need to train the residents, fellows, and other unexperienced doctors. Three different surgical assistants participated in operations during the study period. The assistants participated in incision making and closure and docking, but not robot operation. Therefore, CUSUM analysis based on console time was also performed to reduce the bias caused by different surgical assistants.
Risk-adjusted cumulative sum analysis
Furthermore, risk-adjusted CUSUM (RA-CUSUM) was used to investigate other clinical indicators that may impact the learning curve.21 In this study, surgical failure was defined as any of following events: conversion to open, peri/postoperative complications, and total operative time >180 min. Variables with p < 0.15 in univariate logistic models were included in the multivariate analysis.13 The probability of surgical failure was calculated using a multivariate logistic regression model.13 The RA-CUSUM was defined as RA-CUSUM = 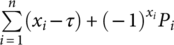 , where xi = 1 indicates the presence of surgical failure, xi = 0 indicates surgical success, τ represents the observed event rate, and Pi represents the expected rate of surgical failure of each case.13
, where xi = 1 indicates the presence of surgical failure, xi = 0 indicates surgical success, τ represents the observed event rate, and Pi represents the expected rate of surgical failure of each case.13
Definition of learning phases
Scatter plots of CUSUM and RA-CUSUM against consecutive cases were depicted and fit with curves based on nonlinear regression (least squares fit method) in GraphPad prism 6.0. Then, we calculated the first derivative of each case in both curves according to the polynomials generated from nonlinear regression to identify the cut-off point, which was defined as the point for which the first derivation equalled zero. Finally, different learning phases were identified based on these cut-off points.
RESULTS
Clinicopathological characteristics of patients
During the study period (June 2018 to August 2019), a total of 161 robotic surgeries were performed by Dr. Hao-Xian Yang and his team. Among these cases, 141 patients underwent lung resections, eight patients underwent esophagectomies, 10 patients underwent mediastinal tumor resections, and two patients underwent mediastinal lymph node dissections. Of the 141 patients who underwent lung resections, 100 patients underwent lobectomies, 24 patients underwent segmentectomies, four patients underwent bilobectomies, two patients underwent pneumonectomies, and 11 patients underwent wedge resections. Table 1 shows the general clinicopathological characteristics of the entire cohort. The mean age of the entire cohort was 56.8 ± 11.1 years (median 59.0 years, range 28–79 years) and 48.0% of the patients were male. Adenocarcinoma (75/100, 75.0%) was the predominant pathological type. Pathological stage I accounted for most of the NSCLC cases (63/84, 75.0%). The mean tumor diameter was 2.3 ± 1.0 cm (median 2.2 cm, range 0.7–7.2 cm). The average total operative time was 130.6 ± 53.8 min (median 120 min, range 47–362 min). Five patients (5%) were converted to an open procedure during surgery. Specifically, four of these cases were due to surgical bleeding, and one case was due to extensive pleural adhesions. No perioperative deaths occurred in this series.
| Characteristics | Number of patients (%) |
|---|---|
| Tumor location | |
| RUL | 37 (37.0) |
| RML | 13 (13.0) |
| RLL | 16 (16.0) |
| LUL | 20 (20.0) |
| LLL | 24 (24.0) |
| Gender | |
| Male | 48 (48.0) |
| Female | 52 (52.0) |
| Age (years) | |
| Mean ± SD | 56.8 ± 11.1 |
| Median (min, max) | 59.0 (28, 79) |
| Tumor size (cm) | |
| Mean ± SD | 2.3 ± 1.0 |
| Median (min, max) | 2.2 (0.7, 7.2) |
| Smoking history | |
| Current | 18 (18.0) |
| Quit | 11 (11.0) |
| Never | 71 (71.0) |
| Neoadjuvant therapy | |
| Yes | 5 (5.0) |
| No | 95 (95.0) |
| Comorbidity | |
| No | 68 (68.0) |
| Yes | 32 (32.0) |
| Anatomical type | |
| Central | 1 (1.0) |
| Peripheral | 99 (99.0) |
| Histology | |
| Adenocarcinoma | 75 (75.0) |
| Squamous cell carcinoma | 5 (5.0) |
| Inflammation | 7 (7.0) |
| Tuberculosis | 2 (2.0) |
| Fungal infection | 4 (4.0) |
| Hamartoma | 1 (1.0) |
| Neuroendocrine carcinoma | 4 (4.0) |
| Pulmonary sclerosing haemangioma | 1 (1.0) |
| Inflammatory myofibroblastic tumor | 1 (1.0) |
| Clinical stage | |
| I | 85 (85.0) |
| II | 5 (5.0) |
| III | 8 (8.0) |
| IVa | 2 (2.0) |
| Pathological stageb | |
| I | 63 (75.0) |
| II | 5 (6.0) |
| III | 13 (15.5) |
| IVc | 3 (3.6) |
| Operative time (min)d | 130.6 ± 53.8 |
| Console time (min)e | 95.5 ± 52.3 |
| Docking time (min) | 6.4 ± 3.0 |
| No. of mediastinal LNs harvested | 17.8 ± 13.5 |
| No. of total LNs harvested | 34.5 ± 18.4 |
| Blood loss (mL) | 116.1 ± 200.9 |
| Cost (¥) | 106234.59 ± 11859.46 |
- Abbreviations: LLL, left lower lobe; LNs, lymph nodes.; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe; SD, standard deviation.
- a Two cases with solitary brain metastasis received brain irradiation before lobectomy.
- b Excluded cases with benign lesions.
- c Two cases with solitary brain metastasis received brain irradiation before lobectomy. Pleural dissemination was found during surgery in one case.
- d Defined as the time of skin to skin. Patients with conversion were excluded.
- e Defined as the time of operating console. Patients converted to open surgery were excluded.
The learning curve
Based on CUSUM analysis of total operative time, the learning curve was fit to a fifth-order polynomial (R2 = 0.7761; Figure 2). Five cases converted to open surgery were excluded from CUSUM analysis. When total operation time (from skin to skin) was used to reflect the learning process, the learning curve was divided into three phases (Figure 2): the learning phase (cases 1–13, phase 1), the plateau phase (cases 14–59, phase 2), and the mastery phase (cases >59, phase 3).
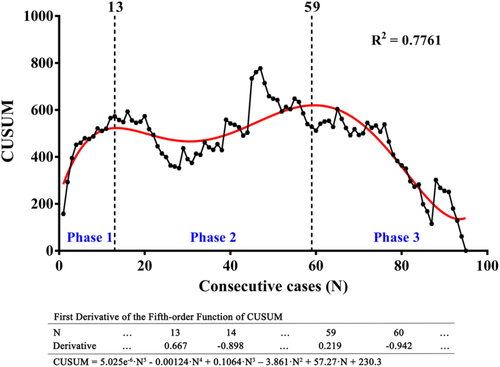
To reduce the bias caused by different surgical assistants, the CUSUM analysis of console time was also performed and the curve was fit to a sixth-order polynomial (R2 = 0.7037; Figure 3). As Figure 3 shows, the learning curve of console time was also divided into three phases: the learning phase (cases 1–10, phase 1), the plateau phase (cases 11–51, phase 2), and the mastery phase (> 51 cases, phase 3).
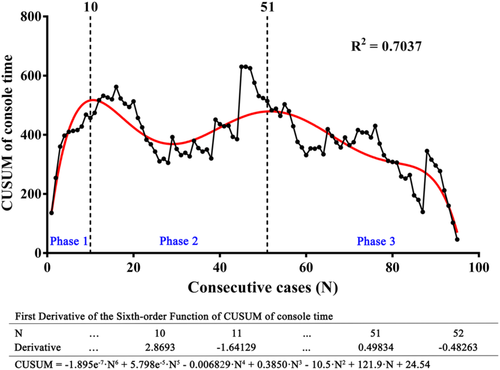
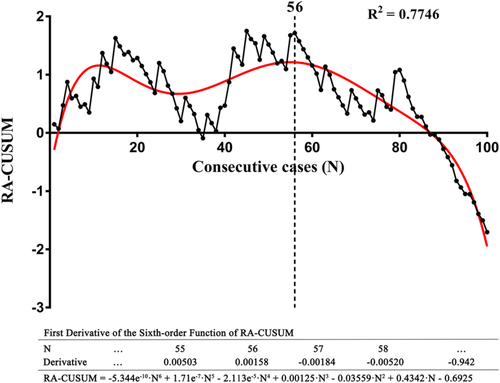
Furthermore, according to univariate and multivariate analyses, surgical failure was only associated with blood loss (Table 2). RA-CUSUM analysis was conducted based on the multivariate analysis, creating a scatter plot and a fit curve (Figure 4). According to Figure 4, the regression curve showed a sustained decline after the 56th case, indicating the decreased possibility of surgical failure. Taken together, the learning curve of RPL-4 can be divided into three phases, and mastery of this technique for acceptable surgical outcomes occurred in phase 2 at the 56th case.
| Characteristics | Success (N = 72) | Failurea (N = 28) | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|---|
| p | B | OR | p | |||
| Sex | 0.844 | |||||
| Male | 35 | 13 | ||||
| Female | 37 | 15 | ||||
| Age (years) | 0.477 | |||||
| ≤ 60 | 42 | 14 | 0.451 | |||
| > 60 | 30 | 14 | ||||
| Smoking history | 0.953 | |||||
| Never | 51 | 20 | ||||
| Quit/current | 21 | 8 | ||||
| Tumor location | 0.771 | |||||
| RUL | 28 | 9 | ||||
| RML | 10 | 3 | ||||
| RLL | 11 | 5 | ||||
| LUL | 13 | 7 | ||||
| LLL | 10 | 4 | ||||
| Tumor size (cm) | 2.3 ± 1.1 | 2.3 ± 1.0 | 0.927 | |||
| Clinical stage | 0.417 | |||||
| I | 63 | 22 | ||||
| II | 3 | 2 | ||||
| III | 5 | 3 | ||||
| IVb | 1 | 1 | ||||
| Comorbidity | 0.985 | |||||
| No | 49 | 19 | ||||
| Yes | 23 | 9 | ||||
| Histology | 0.552 | |||||
| Benign | 59 | 25 | ||||
| Malignant | 13 | 3 | ||||
| Blood loss (ml) | 50 (50.0, 60.0) | 100 (62.5, 300.0) | <0.001 | 0.021 | 1.021 | 0.001 |
| No. of mediastinal LNs harvested | 17.7 ± 13.1 | 18.1 ± 14.9 | 0.923 | |||
| Constant | −2.864 | 0.057 | <0.001 | |||
- Abbreviations: LLL, left lower lobe; LNs, lymph nodes.; LUL, left upper lobe; OR, odds ratio; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
- a Surgical failure was defined as any of following events: conversion to open, perioperative complications, and operative time >180 min.
- b Two cases with solitary brain metastasis received brain irradiation before lobectomy.
Comparison among learning phases of console time
The comparison of clinicopathologic characteristics is shown in Table 3. All the clinicopathologic characteristics were well balanced among the three phases (Table 3). The percentages of upper lobectomies were 60%, 46.3%, and 61.4% for phase 1, phase 2, and phase 3, respectively, with no statistically significant difference (p = 0.055; Table 3). The percentages of N1 diseases were 0, 12.1%, and 2.6% for phase 1, phase 2, and phase 3, respectively, without statistically significant difference (p = 0.346; Table 3). The only case with a central tumor was in phase 2 and the numbers of patients with infectious diseases (including inflammation, tuberculosis, and fungal infection) were 2, 6, and 5 for phase 1, phase 2, and phase 3, respectively (Table 3). The comparison of perioperative outcomes between different phases is shown in Table 4. As predicted, reductions in total operative time (p < 0.001), console time (p < 0.001), and docking time (p = 0.026) were observed over the three phases of the learning curve. Other outcomes were all comparable between different phases. Comparison between each two adjacent phases indicated that total operative time, console time, and docking time showed a difference between phases 1 and 2 (p = 0.005, p = 0.003, p = 0.012, respectively), but this difference was only observed for total operative time between phases 2 and 3 (p = 0.044, p = 0.145, p = 0.216, respectively). However, the rates of postoperative complication in phases 1 and 2 were up to 10.0% and 12.2%, respectively, but this rate decreased to 4.5% in phase 3.
| Characteristics | Phase 1 (N = 10) | Phase 2 (N = 41) | Phase 3 (N = 44) | p |
|---|---|---|---|---|
| Tumor location | 0.055 | |||
| RUL | 1 (10.0) | 16 (39.0) | 18 (40.9) | |
| RML | 1 (10.0) | 9 (22.0) | 3 (6.8) | |
| RLL | 2 (20.0) | 7 (17.1) | 7 (15.9) | |
| LUL | 5 (50.0) | 3 (7.3) | 9 (20.5) | |
| LLL | 1 (10.0) | 6 (14.6) | 7 (15.9) | |
| Gender | 0.939 | |||
| Male | 5 (50.0) | 20 (48.8) | 20 (45.5) | |
| Female | 5 (50.0) | 21 (51.2) | 24 (54.5) | |
| Smoking history | 0.226 | |||
| Current | 1 (10.0) | 5 (12.2) | 11 (25.0) | |
| Quit | 2 (20.0) | 3 (7.3) | 5 (11.4) | |
| Never | 7 (70.0) | 33 (80.5) | 28 (63.6) | |
| Neoadjuvant therapy | 1.000* | |||
| Yes | 0 | 1 (2.4) | 4 (9.1) | |
| No | 10 (100.0) | 40 (97.6) | 40 (90.9) | |
| Comorbidity | 0.773 | |||
| No | 7 (70.0) | 26 (63.4) | 31 (70.5) | |
| Yes | 3 (30.0) | 15 (36.6) | 13 (29.5) | |
| Age (years) | 52.9 ± 14.2 | 59.6 ± 10.1 | 54.3 ± 10.7 | 0.050 |
| Tumor size (cm) | 2.8 ± 1.7 | 2.2 ± 1.1 | 2.2 ± 0.8 | 0.482 |
| Anatomical type | 1.000* | |||
| Central | 0 | 1 (2.4) | 0 | |
| Peripheral | 10 (100.0) | 40 (97.6) | 44 (100.0) | |
| Histology | 0.068 | |||
| Adenocarcinoma | 5 (50.0) | 31 (75.6) | 37 (84.1) | |
| Squamous cell carcinoma | 1 (10.0) | 2 (4.9) | 1 (2.3) | |
| Inflammation | 0 | 5 (12.2) | 2 (4.5) | |
| Tuberculosis | 1 (10.0) | 0 | 1 (2.3) | |
| Fungal infection | 1 (10.0) | 1 (2.4) | 2 (4.5) | |
| Hamartoma | 0 | 1 (2.4) | 0 | |
| Neuroendocrine carcinoma | 1 (10.0) | 0 | 1 (2.3) | |
| Pulmonary sclerosing haemangioma | 0 | 1 (2.4) | 0 | |
| Inflammatory myofibroblastic tumor | 1 (10.0) | 0 | 0 | |
| Clinical stage | 0.827 | |||
| I | 8 (80.0) | 35 (87.5) | 38 (86.4) | |
| II | 0 | 3 (7.5) | 2 (4.5) | |
| III | 2 (20.0) | 2 (5.0) | 3 (6.8) | |
| IVb | 0 | 0 | 1 (2.3) | |
| Pathological stagec | 0.245 | |||
| I | 7 (100.0) | 23 (69.7) | 29 (74.4) | |
| II | 0 | 4 (12.1) | 1 (2.6) | |
| III | 0 | 4 (12.1) | 8 (20.5) | |
| IVd | 0 | 2 (6.1) | 1 (2.6) | |
| Pathological T categoryc | 0.548 | |||
| T1 | 4 (57.1) | 24 (72.7) | 30 (76.9) | |
| T2 | 3 (42.9) | 8 (24.2) | 8 (20.5) | |
| T3 | 0 | 1 (3.0) | 1 (2.6) | |
| T4 | 0 | 0 | 0 | |
| Pathological N categoryc | 0.346 | |||
| N0 | 7 (100.0) | 25 (75.8) | 30 (76.9) | |
| N1 | 0 | 4 (12.1) | 1 (2.6) | |
| N2 | 0 | 4 (12.1) | 8 (20.5) |
- Abbreviations: LLL, left lower lobe; LUL, left upper lobe; RLL, right lower lobe; RML, right middle lobe; RUL, right upper lobe.
- a Excluded five cases converted to open surgery.
- b Two cases with solitary brain metastasis received brain irradiation before lobectomy.
- c Excluded cases with benign lesions.
- d Two cases with solitary brain metastasis received brain irradiation before lobectomy. Pleural dissemination was found during surgery in one case.
- * Fisher's exact test.
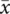 ±s)
±s)| Outcomes | Phase 1 (N = 10) | Phase 2 (N = 41) | Phase 3 (N = 44) | Total (N = 95) | pb |
|---|---|---|---|---|---|
| Total operative timec (min) | 181.7 ± 60.1 | 133.85 ± 52.5 | 116.1 ± 46.6 | 130.6 ± 53.8 | 0.001 |
| Console timed (min) | 140.7 ± 53.9 | 96.4 ± 53.0 | 84.4 ± 46.3 | 95.5 ± 52.3 | 0.001 |
| Docking time (min) | 9.5 ± 5.2 | 5.7 ± 2.4 | 6.2 ± 2.4 | 6.3 ± 3.0 | 0.026 |
| Blood loss (ml) | 93.0 ± 52.1 | 80.4 ± 73.8 | 72.3 ± 64.3 | 78.0 ± 67.1 | 0.238 |
| No. of mediastinal LNs harvested | 15.7 ± 11.9 | 19.3 ± 17.3 | 17.5 ± 10.1 | 18.1 ± 13.7 | 0.743 |
| No. of total LNs harvested | 29.3 ± 12.9 | 38.2 ± 22.7 | 33.0 ± 15.3 | 34.8 ± 18.8 | 0.513 |
| Conversione, % | 9.1 (1/11) | 4.7 (2/43) | 4.3 (2/46) | 5.0 (5/100) | 1.000* |
| Postoperative complication, % | 10.0 (1/10) | 12.2 (5/41) | 4.5 (2/44) | 8.4 (8/95) | 1.000* |
| Atrial fibrillation | 10.0 (1/10) | 2.4 (1/41) | 2.3 (1/44) | 3.2 (3/95) | |
| Air leak >5 days | 0 | 7.3 (3/41) | 2.3 (1/44) | 2.1 (2/95) | |
| Atelectasis | 0 | 2.4 (1/41) | 0 | 3.2 (3/95) | |
| Chest tube duration (days) | 3.4 ± 0.8 | 3.9 ± 1.6 | 3.7 ± 1.2 | 3.7 ± 1.4 | 0.701 |
| Length of stay in hospital (days) | 5.0 ± 0.7 | 5.1 ± 1.5 | 4.7 ± 1.1 | 4.9 ± 1.2 | 0.490 |
| Cost (¥) | 102151.7 ± 7793.7 | 105871.2 ± 14976.7 | 107308.8 ± 9618.0 | 106145.5 ± 12080.7 | 0.254 |
- Abbreviation: LNs, lymph nodes.
- a Excluded five cases converted to open surgery.
- b Compared among three phases.
- c Defined as the time of skin to skin.
- d Defined as the time of operating console.
- e No. of patients converted to open surgery divided by no. of total patients.
- * Fisher's exact test.
DISCUSSION
RATS is a rapidly developing technique in minimally invasive thoracic surgery. Like other new surgical technologies, the learning process requires time and volume. How to overcome the learning curve safely and quickly is a key point of consideration by any surgeon planning to adopt RATS. Unfortunately, the process by which a surgeon learns a new technique can be subjective and difficult to define. The purpose of this study is to objectify the subjective process of learning the RPL-4 and share our experience with other surgeons. This study is unique because it is a prospective observational study that is specific to a single type of operation (RPL-4), and a single surgical team in a single hospital performed the surgeries using a single robot.
In this study, we accumulated the data from the first 100 consecutive cases undergoing RPL-4 in our center. The data suggested that the learning curve for console operation could be divided into three phases: the learning phase (phase 1, case 1–10), the plateau phase (phase 2, case 11–51), and the mastery phase (phase 3, > 51 cases). As predicted, reduced operation time was observed as operation experience accumulated. RA-CUSUM analysis suggested that at least 56 cases were required to guarantee satisfactory surgical outcomes of the RPL-4 procedure, indicating mastery of this technique.
Meyer et al. performed a retrospective study including 185 cases with robotic lobectomy from January 2004 to December 2011.11 They used three arms directly placed through three 2-cm incisions without using any trocars, and they referred to this procedure as the nontrocar technique.22 Their data suggested that approximately 18 cases were required to pass the learning curve. Fahim et al. performed a similar study, and 20 cases were required to master the RATS technique in lung resection.12 Arnold et al. conducted a CUSUM analysis including 101 patients undergoing robotic lobectomy using four arms of the Da Vinci Si system combined with a 3-cm assistant's port.10 This operative technique was referred to as robot assisted lobectomy (RAL-4) based on the recently suggested nomenclature.15, 16 The study revealed that the learning curve could be divided into three phases (cases 1–22, cases 23–63, and cases 64–101). In our study, the case number for the learning phase is 10, which is less than in these previous studies.10-12 There are some reasons explaining this difference. First, the prospectively collected data of this study makes the outcomes more reliable. Second, the attending surgeon in this study was an experienced surgeon in VATS procedure, and the rich experience in VATS may help the surgeon to cross the learning curve of RPL-4 earlier.23 Third, the techniques we used, including the use of all the four arms of the robot, the use of CO2 insufflation, and the operation ports' setting, were suitable for learning RATS. The ports setting in this study originated from Cerfolio RJ's procedure6 but we modified it to adapt to Chinese patients. Unlike Caucasian people, Asian people are generally small in stature. The space is usually not large enough to set all the four ports in one intercostal space (the eighth intercostal space) in Chinese patients. We therefore set the anterior port to the fifth or sixth intercostal space to avoid obstruction between instruments. In addition, the location of the assistant port was not consistent for all patients but depended on the tumor location because pulmonary vessels of different lobes had different directions. For tumors in the upper or middle lobes, the assistant port was set in the 10th intercostal space between the posterior axillary line and the tip of the scapular line. For lower lobe tumors, the assistant port was set in the eighth intercostal space anterior to the camera port for at least 8 cm. This strategy had proved to be very effective and safe in the transection of pulmonary vessels for surgical assistant.
In this study, the number of dissected LNs, postoperative complications, blood loss, chest tube duration, the length of hospital stay, and cost were comparable among the three phases. These results are similar to other previous retrospective studies.10, 12, 14 These outcomes suggest that the principle of surgical oncology was not compromised at the beginning of the learning curve in our series. However, it should be noted that the rate of postoperative complication in phase 3 was lower than that in phases 1 and 2. When the plateau phase (phase 2) passed, the surgeon mastered the technique proficiently, and the rate of postoperative complication subsequently decreased. RA-CUSUM analysis suggested that at least 56 cases were required to guarantee satisfactory perioperative outcomes. Therefore, we proposed that the mastery of RPL-4 for satisfactory surgical outcomes requires experience from 56 cases.
It is interesting that the docking time seemed longer in phase 3 than in phase 2. As a university hospital, we train fellows and residents. In phases 1 and 2, the attending surgeon participated in the docking work for most of the cases. In phase 3, however, the fellows performed a considerable amount of the docking work. This notion can explain the difference in docking time between phases 2 and 3. To reduce the bias caused by surgical assistants, we conducted another CUSUM analysis based on the console time but not the time from skin to skin. As predicted, the data based on console time suggested that fewer cases were needed to pass the learning curve, but it should be noted that other robotic procedures, such as esophagectomies (eight cases), wedge resections (11 cases), segmentectomies (24 cases), bilobectomies (four cases), pneumonectomies (two cases), and surgeries for mediastinal tumors (10 cases) were also performed by this surgical team during the study period. This may also impact the learning curve.
Some limitations need to be considered when interpreting our data. First, this was a single-institutional study in which the operations were performed by one surgical team. It should be noted that the robotic surgical team in this study was led by a senior thoracic surgeon who is experienced in VATS. The findings of this study may therefore vary with other surgical teams. However, we think that this feature also increases the homogeneity of the data. Homogeneity in learning curve analysis is also important because the data demonstrate one surgeon's learning process and are not confounded by other surgeons' experiences. Second, this study only included the first 100 cases of the RPL-4 procedure. The learning curve of lobectomy for individual lobes may be different. However, the case number is not large enough to conduct subgroup analysis based on tumor location. The learning curve may be impacted by the case number, and further data are essential to update the outcomes.
In conclusion, our data suggest that the learning curve of RPL-4 can be divided into three phases. Ten cases were required to pass the learning curve, but mastery of RPL-4 for satisfactory surgical outcomes requires experience from at least 56 cases.
ACKNOWLEDGEMENTS
This work was supported in part by the Natural Science Foundation of Guangdong Province (2018A030313410, 2020A151501311), the Sun Yat-sen University Clinical Research 5010 Program (2019012), and the National Natural Science Foundation of China (82072572).
CONFLICT OF INTEREST
The author has no conflicts of interest to declare.