MicroRNA-210 targets FBXO31 to inhibit tumor progression and regulates the Wnt/β-catenin signaling pathway and EMT in esophageal squamous cell carcinoma
Abstract
Evidence from previous studies showed that the dysregulation of microRNA (miR) is frequently associated with tumor progression. The aberrant miR-210 expression has been identified in a variety of tumors. However, its biological roles in esophageal squamous cell carcinoma (ESCC) still need further elucidation. Thus, in the current study we explore the roles of miR-210 in ESCC progression. The findings of our study reveal that miR-210 is down-regulated in ESCC, which indicates poor prognosis and aggressive tumor progression. Moreover, miR-210 restoration was found to enhance ESCC viability, invasion, and migration abilities. F-Box only protein 31 (FBXO31) was confirmed to be one of the targets of miR-210 in ESCC cells. Results also revealed that miR-210 played crucial roles in regulating ESCC cell epithelial-mesenchymal transition (EMT) and Wnt/β-catenin signaling. In conclusion, data show that miR-210 serves as an anti-ESCC miR via down-regulation of FBXO31 and regulation of EMT and Wnt signaling, suggesting that the miR-210/FBXO31 axis may function as promising therapeutic targets and effective prognostic markers for ESCC patients.
INTRODUCTION
Esophageal carcinoma (EC) is one of the most lethal malignancies worldwide, with high morbidities and mortalities.1, 2 Histologically, esophageal adenocarcinoma and esophageal squamous cell carcinoma (ESCC) are the main types of EC, accounting for 90% of all EC cases.3 In recent years, prognosis has been improved thanks to the combination of radiotherapy and chemotherapy, which can be applied alone or as a surgical adjunct regimen.4 However, the prognosis of ESCC patients still remains unsatisfactory due to the fact that tumors are inoperable in many cases and surgery is only feasible in limited patients.5 Although epigenetic and genetic changes are the basis of ESCC development, the mechanism underlying ESCC progression needs further elucidation.6, 7 Cumulative evidence has indicated that a variety of tumor-suppressive and oncogenic genes are implicated in ESCC genesis and development.8 Therefore, it is critical to explore the potential mechanisms of ESCC for the development of more effective diagnostic and therapeutic targets.
MicroRNA (miRNA/miR) is a small noncoding RNA (18–25 nucleotides in length) that has been reported to regulate gene expressions by binding to the 3′-untranslated region (UTR) of the target mRNAs, resulting in translational repression or degradation.9 A growing number of studies have demonstrated the regulatory roles of miRs in cell metastasis, apoptosis, proliferation, and differentiation in a variety of malignancies, highlighting the potential of miRs in the diagnosis, therapies, and prognosis predictions of tumors.10-12 Various novel and highly sensitive miRs are identified in predicting the prognosis of ESCC. For instance, miR-155 was reported to act as a diagnostic and prognostic biomarker for ESCC,13 miR-301a-3p regulated the esophageal squamous cell proliferation via targeting Phosphatase and tensin homolog deleted on chromosome 10 (PTEN),14 miR-25-3p was found to target PTEN to regulate esophageal cancer cell migration, invasion, and apoptosis via the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) pathway,15 and serum exosomal miR-766-3p expression was associated with poor prognosis of ESCC.16 Taken together, all these findings reveal that application of specific miRs based on the dysregulation of them can assist early diagnosis and prognosis of patients with ESCC. In recent years, a growing body of experimental evidence has suggested a clinical relevance for miR-210. The functions of miR-210 have been studied in various cancers and the up-regulation of miR-210 has been identified in most solid tumors, which was correlated with a negative clinical outcome.17, 18 Moreover, miR-210 was reported to have relatively moderate accuracy in distinguishing different cancer patients from all other individuals, providing comprehensive and synthetic evidence for miR-210 as a potential noninvasive biomarker in cancer detection and diagnosis.19 In ESCC, studies by Soken Tsuchiya showed that the expression of miR-210 in ESCC was down-regulated and miR-210 could inhibit ESCC cell proliferation via targeting fibroblast growth factor receptor like 1 (FGFRL1).20 However, the precise functions and mechanisms in ESCC should be further explored. In the current study, we further investigate the clinical values of miR-210 in ESCC. F-Box only protein 31 (FBXO31) is a member of F-box protein family, which contains an F-box motif.21 FBXO31 functions as a substrate-recognition component in the ubiquitin proteasome system, mediating degradation of target protein.22 It has been reported that FBXO31 is implicated in DNA damage response through regulating the degradation of cyclin D1 (CCND1) via the ubiquitin proteasome pathway and thereby inducing G1 arrest.23 Moreover, a large array of studies has indicated that FBXO31 plays a crucial role in tumorigenesis. In gastric cancer, FBXO31 could suppress epithelial-mesenchymal transition (EMT) via regulation of Snail1 for proteasomal degradation.24 Down-regulated FBXO31 in hepatocellular carcinoma functions as a tumor suppressor.25 Additionally, FBXO31 could promote lung cancer cell growth, invasion, and metastasis.26 EMT describes a series of events during which cells lose epithelial characteristics such as cell-layer organization and apical-basolateral polarization, and acquire properties of mesenchymal or fibroblastoid cells, including motility.27 EMT is known to be a central mechanism responsible for the invasiveness and metastasis of various cancers.28 In this study, we investigated the correlation between FBXO31 and miR-210 in ESCC progression.
MATERIALS AND METHODS
Patients and tissues
Paired ESCC tissue specimens and matched nontumor esophageal squamous tissue samples were collected from ESCC patients at the Rizhao Hospital of Traditional Chinese Medicine. No patient had received preoperative radiotherapy or chemotherapy. Each patient provided written informed consent. All tissues were flash-frozen in liquid nitrogen and stored at −80°C. The current study received approval from the Ethical Review Committee of the Rizhao Hospital of TCM.
Cell culture and cell transfection
Human ESCC cell lines (EcA109 and TE1) and normal esophageal epithelial cell line (HET-1A) were purchased from the BeNa Culture Collection (Jiangsu, China). The above cell lines were maintained in Roswell Park Memorial Institute Medium (RPMI-1640 medium; Thermo Fisher Scientific Inc.). The medium contained 10% fetal bovine serum (FBS; Gibco) and cells were incubated in a humidified chamber with 5% CO2 at 37°C. MiR-210 mimic, inhibitor, small interfering RNA (siRNA) against FBXO31 (si- FBXO31) and the negative control (NC) were commercially synthesized by GenePharma. The transfections were performed by lipofectamine 2000 (Invitrogen).
qRT-PCR
Extraction of total RNAs from ESCC cell lines or tissue samples was performed by TRIzol reagent (Thermo Fisher Scientifc, Inc.). Following cDNA synthesis with the PrimeScript™ RT-PCR kit (Takara Biotechnology Co., Ltd), FBXO31 and miR-210 expressions were examined with SYBR Premix ExTaq (Takara Biotechnology Co. Ltd) reagents on the ABI 7500 System (Applied Biosystems). The primer is shown in Table 1. Relative miRNA and mRNA expressions were normalized with U6 and GAPDH, and the relative quantifications were conducted using the 2-ΔΔCt method.
| Primer | Sequence |
|---|---|
| miR-210 forward | 5′-CATAGATAGCCACTGCCCACA −3′ |
| miR-210 reverse | 5′- GTGCAGGGTCCGAGGTATTC-3′ |
| U6 forward | 5′- CTCGCTTCGGCAGCACA-3′ |
| U6 reverse | 5′- AACGCTTCACGAATTTGCGT-3′ |
| FBXO31 forward | 5′- CCGGCGGGAGGCAGGAGGAGT −3′ |
| FBXO31 reverse | 5′- GCGGCGGTAGGTCAGGCAGTTGTCG −3′ |
| GAPDH forward | 5′- TAATCTTCGCCTTAATACTT-3′ |
| GAPDH reverse | 5′- AGCCTTCATACATCTCAA-3′ |
- Abbreviations: FBXO31, F-Box only protein 31; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; snRNA, U6 small nuclear RNA.
Western blots
Isolation of total proteins from EcA109 cell lines (5 × 106 cells) was performed by RIPA lysis buffer (Beyotime). The protein concentrations were quantified by bicinchoninic acid (BCA) Protein Assay (Beyotime). Equal protein samples were separated by 10% Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membrane (Millipore). Having been blocked with 5% skimmed milk for 2 h at room temperature, the membrane was incubated with specific primary antibodies overnight at 4 °C. Finally, following incubation with the corresponding secondary antibody (1:4000; Abcam), the signals were detected by an enhanced chemiluminescence kit (Millipore). Primary antibodies used in the western blot analysis were antibodies against cyclin D1 (1:1000), c-Myc (1:1000), β-catenin (1:1000), E-cadherin (1:2000), N-cadherin(1:2000), vimentin (1:1000) and GAPDH (1:1000), which were all obtained from Abcam. GAPDH was an internal reference.
Viability assays
Transfected EcA109 cells (2 × 103) were placed into 96-well plates and cultivated for designated time intervals of 24, 48 and 72 h. MTT assays were performed by adding MTT solution (Sigma-Aldrich) to the cells and incubating at 37°C for 4 h. After removing the culture medium, DMSO (Sigma-Aldrich) was then added to each well to solubilize the formazan crystals. Finally, OD490 was examined with a microplate reader (BioTek).
Transwell assay
The transwell chamber (8 μm diameter; Corning Inc.) was utilized to detect the migration and invasion capacities of the transfected EcA109 cells. Briefly, the transfected cells (3 × 105) resuspended in serum-free medium were seeded in the top compartment of the chamber. Medium containing 10% FBS, which served as a chemoattractant, was added to the bottom compartment. After incubation for 48 h, cells on the bottom surface of the membrane were fixed and stained. Finally, the migratory cells were quantified under a light microscope (Olympus). For cell invasion assays, a similar insert coated with Matrigel (Corning) was applied to detect cell invasion according to the above procedures.
Dual-luciferase report assay
The FBXO31 3′-UTR fragments with the wild-type (WT) or mutant-type (MUT) putative binding sites of miR-210 were cloned into pGL3 vectors (Promega). The ESCC cells were cotransfected with FBXO31 3′-UTR-WT/MUT and miR-210 mimics/NC by Lipofectamine 2000 (Invitrogen). Forty-eight hours after the transfections, ESCC cells were harvested and detected using the Dual-Luciferase Reporter assay system (Promega).
Statistical analysis
All experiments were performed in triplicate. SPSS 16.0 (SPSS Inc.) was used to perform the statistical analysis. Statistical significance was analyzed with Student's t-test or one-way analysis of variance followed by a Tukey's post hoc test. The prognosis of ESCC patients was examined using Kaplan–Meier analysis together with the log-rank test. p < 0.05 indicated statistically significant differences.
RESULTS
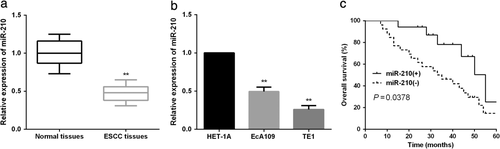
Down-regulated miR-210 correlated with aggressive ESCC progression. To explore the clinicopathologic value of miR-210 in ESCC patients, qRT-PCR assays were performed to measure miR-210 expression levels in ESCC tissue samples and cell lines. The results showed that miR-210 was remarkably underexpressed in the collected ESCC tissue samples compared to the matched normal tissues (Figure 1(a)). Similarly, the prominently decreased miR-210 expression was also confirmed in ESCC cell lines (Figure 1(b)). Subsequently, all the enrolled patients were assigned into a high or low miR-210 group based on the mean miR-210 level to investigate its clinicopathologic significance in ESCC patients. As shown in Figure 1(c), Kaplan–Meier survival analysis demonstrated a significant poor prognosis in ESCC patients with low miR-210 level. Moreover, decreased miR-210 was also found to correlate with malignant clinicopathologic parameters of ESCC patients (Table 2).
| Clinicopathological features | Cases (n = 43) | miR-210b expression | p value | |
|---|---|---|---|---|
| High (n = 17) | Low (n = 26) | |||
| Age (years) | 0.613 | |||
| >60 | 22 | 8 | 14 | |
| ≤60 | 21 | 9 | 12 | |
| Gender | 0.512 | |||
| Male | 22 | 7 | 15 | |
| Female | 21 | 10 | 11 | |
| Tumor size (cm) | 0.345 | |||
| ≥ 5.0 | 22 | 8 | 14 | |
| <5.0 | 21 | 9 | 12 | |
| TNM stage | 0.023a | |||
| I-II | 18 | 14 | 4 | |
| III | 25 | 3 | 22 | |
| Lymph-node metastasis | 0.018a | |||
| Yes | 24 | 4 | 20 | |
| No | 19 | 13 | 6 | |
| Tumor location | ||||
| Upper | 14 | 7 | 7 | 0.536 |
| Middle | 14 | 5 | 9 | |
| Lower | 15 | 5 | 10 | |
| Differentiation | 0.011a | |||
| Well and moderate | 19 | 14 | 5 | |
| Poorly and not | 24 | 3 | 21 | |
| Clinical stage | 0.006a | |||
| I-II | 35 | 15 | 20 | |
| III-IV | 8 | 2 | 6 | |
- Abbreviations: ESCC, esophageal squamous cell carcinoma; TNM, tumor-node-metastasis.
- a Statistically significant.
- b The median expression level of miR-210 was used as the cutoff.
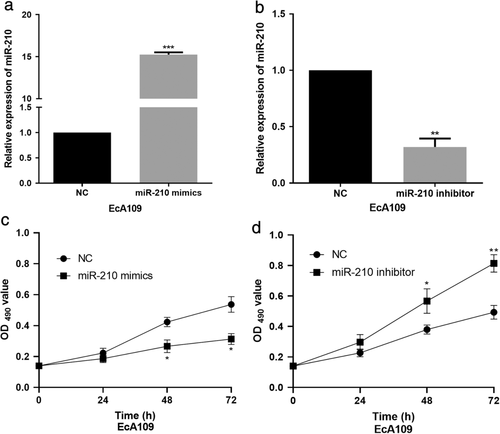
MiR-210 up-regulation suppressed ESCC cell viability. The EcA109 cell line was selected to perform the gain or loss assays due to its relatively higher endogenic miR-210 expression. To elucidate the functions of miR-210 in ESCC progression, miR-210 mimics or inhibitor was transfected into EcA109 cells. The efficient overexpression or inhibition of miR-210 was confirmed by qRT-PCR (Figure 2(a),(b)). MTT assay was carried out to determine the roles of miR-210 in EcA109 cell proliferation. The data revealed that miR-210 overexpression could obviously repress the proliferation ability of EcA109 cells (Figure 2(c)) while miR-210 silence evidently enhanced EcA109 cell proliferation (Figure 2(d)).
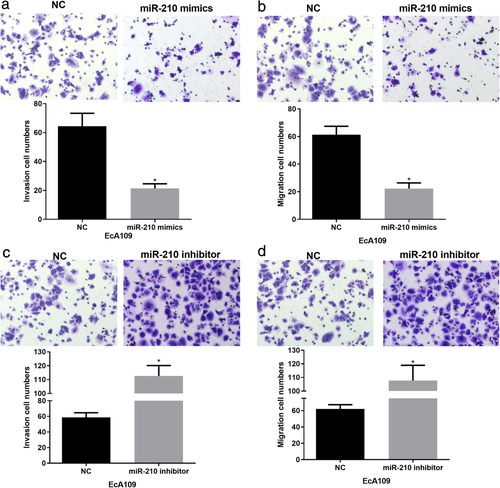
MiR-210 up-regulation inhibited ESCC cell invasion and migration. Considering the implication of miR-210 in ESCC cell proliferation, we next performed transwell assays to explore its roles in ESCC cell invasion and migration. It was shown that the invasion and migration abilities of EcA109 cells were significantly reduced by miR-210 mimics (Figure 3(a),(b)). Conversely, in the miR-210 inhibitor group, the invasion and migration abilities were dramatically increased compared to the control group (Figure 3(c),(d)). Collectively, all these data indicate that miR-210 suppressed the aggressive ESCC progression.
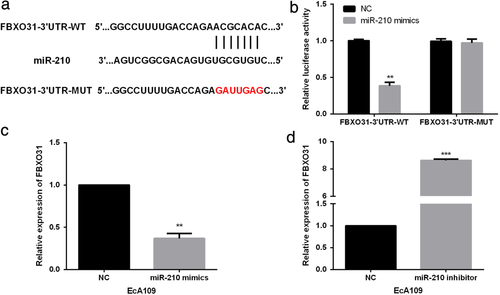
MiR-210 regulated FBXO31 expressions in ESCC cells. To elucidate the underlying mechanism of the suppressive roles triggered by miR-210 in ESCC, TargetScan was used to explore the potential targets of miR-210 and results showed that FBXO31 was a candidate target of miR-210 (Figure 4(a)). Subsequently, luciferase reporter assay was performed by cotransfecting miR-210 mimics/NC and FBXO31-3′UTR-WT/MUT into EcA109 cells. The results revealed that the luciferase activity of the FBXO31-3′UTR-WT was dramatically reduced by miR-210 mimics whereas the luciferase activity in FBXO31-3′UTR-MUT group was not obviously affected by miR-210 mimics (Figure 4(b)), indicating that FBXO31 was a target of miR-210. qRT-PCR was further carried out to validate the correlation between FBXO31 and miR-210. We found that FBXO31 expressions in miR-210 mimics-transfected EcA109 cells were significantly down-regulated (Figure 4(c)). On the other hand, miR-210 inhibitor evidently up-regulated the FBXO31 levels in EcA109 cells (Figure 4(d)).
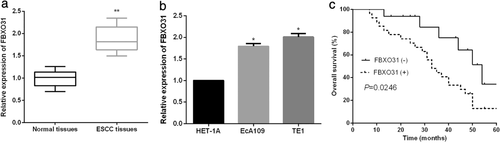
Up-regulated FBXO31 in ESCC correlated with poor clinical prognosis of ESCC patients. We measured the FBXO31 expression levels in ESCC tissues and cells. As demonstrated by qRT-PCR analysis, FBXO31 expressions were significantly increased in ESCC tissues and cells (Figure 5(a),(b)). Thereafter, the prognostic value of FBXO31 in ESCC patients was further investigated. As shown in Figure 5(c), patients with high FBXO31 expressions had a shorter overall survival rate than those with low FBXO31 expressions.
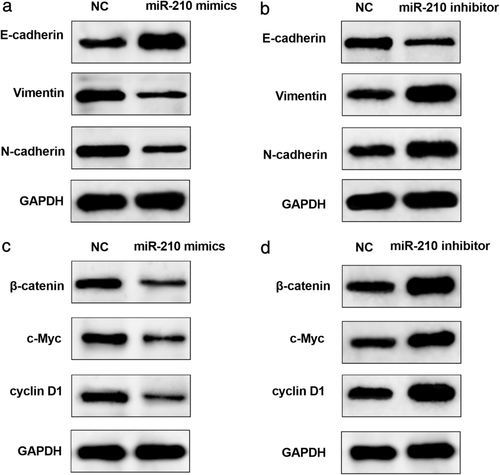
MiR-210 regulated EMT and Wnt/β-catenin signaling pathway in ESCC cells. Western blot was conducted to detect the expression levels of related proteins involved in EMT and Wnt signaling to clarify the mechanisms of the anti-ESCC roles mediated by miR-210. We first examined the expressions of EMT-associated proteins. In EcA109 cells, miR-210 overexpression significantly increased the expressions of E-cadherin and decreased the expression levels of vimentin and N-cadherin(Figure 6(a)). On the other hand, E-cadherin was remarkably down-regulated whereas vimentin and N-cadherin were dramatically up-regulated by miR-210 inhibition in EcA109 cells (Figure 6(b)). Similarly, the regulatory roles of miR-210 in the Wnt/β-catenin signaling pathway of ESCC cells were also investigated. In miR-210-overexpressed EcA109 cells, expressions of activated β-catenin, c-Myc, and cyclin D1 were prominently reduced (Figure 6(c)). Conversely, there was a significant increase of the miR-210-suppressed EcA109 cells (Figure 6(d)). These data reveal that miR-210 exerted its functions in ESCC cells via blocking of EMT and the Wnt/β-catenin signaling pathway.
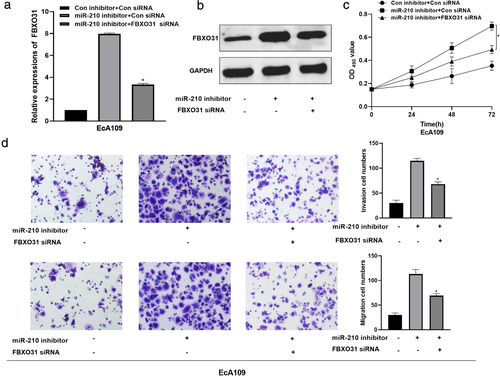
FBXO31 inhibition relieved the miR-210-inhibitor-induced effects on ESCC cells. The regulatory relation between miR-210 and FBXO31 was investigated by performing rescue assays after transfection with miR-210 inhibitor, miR-210 inhibitor + si-FBXO31 or respective controls. First, we observed that the promotion of FBXO31 expression induced by miR-210 inhibitor was abated through FBXO31 knockdown (Figure 7(a),(b)). The results of the CCK-8 assay indicated that the intervention of miR-210 evoked ESCC cell viability, which was abolished by the silence of FBXO31 (Figure 7(c)). Additionally, FBXO31 silence abrogated the promotion of cell invasion and migration caused by miR-210 inhibitor (Figure 7(d)). Taken together, these results demonstrate that FBXO31 reversed the miR-210 inhibitor-induced promotion of cell viability, invasion, and migration in ESCC cells.
DISCUSSION
As ESCC occurrence and development is a complicated process, a thorough understanding of the precise mechanism underlying ESCC development is of great significance. Increasing evidence has demonstrated that miRs are involved in tumor genesis and progression.29-31 Therefore, investigations of tumor-specific miRs could contribute to identifying promising biomarkers and therapeutic targets for tumor therapies. So far, correlation analysis between ESCC and miRs has demonstrated a series of dysregulated miRs, which correlate with the outcomes of ESCC patients.32 For instance, miR-455-3p inhibited ESCC cell proliferation and invasion, and served as a prognostic marker.33 MiR-874-3p was an antitumor miR and independent prognostic factor in ESCC.34 Up-regulation of miR-765 in ESCC predicted a poor prognosis.35
MiR-210 is an important regulator of various tumors, and aberrant miR-210 expression has been identified in a variety of tumors. MiR-210 facilitated lung adenocarcinoma growth, invasion, and metastasis via regulation of targeting lysyl oxidase-like 4.36 Up-regulation of miR-210 inhibited glioma cell growth and enhanced apoptosis through regulating switch-insensitive 3 transcription regulator family member A (SIN3A).37 In the current study, we found that miR-210 was dramatically underexpressed in ESCC and decreased miR-210 expression was verified to correlate with aggressive tumor progression of ESCC. Moreover, miR-210 prominently repressed ESCC cell growth, invasion, and metastasis as demonstrated by functional assays. ESCC tumorigenesis is a complex network containing numerous tumor suppressors, oncogenes, as well as their regulators. In our study, FBXO31 was verified to be a functional target of miR-210, and participated in the suppressive functions of miR-210 in ESCC.
EMT involves the conversion of epithelial cells into mesenchymal cells, and is characterized by increased motility and decreased intracellular adhesion.38 Cumulative evidence has shown that EMT plays an integral role in tumor invasion and metastasis, including ESCC.39 The EMT process comprises many molecular and cellular events, accompanied by the gain of mesenchymal marker (such as Vimentin and N-cadherin) and loss of epithelial marker (such as E-cadherin). Wnt/β-catenin signaling pathway is an important cascade mediating human cancer development, especially cell proliferation.40 Previous studies showed that Wnt signaling was associated with ESCC tumorigenesis.41 Consistent with the above studies, we found that miR-210 overexpression inhibited EMT and inactivated Wnt signaling in ESCC.
In conclusion, the down-regulation of miR-210 in ESCC was correlated with aggressive tumor progression and poor prognosis of ESCC patients. Moreover, as demonstrated by functional assays, miR-210 up-regulation could restrain ESCC cell growth, invasion, and migration via inhibiting EMT and blocking Wnt signaling. Additionally, we also identified FBXO31 as a target of miR-210 and it may partially regulated the suppressive functions of ESCC mediated by miR-210.




