Transformation from adenocarcinoma to squamous cell lung carcinoma with MET amplification after lorlatinib resistance: A case report
Abstract
To date, several studies have described the mechanism of resistance to first- or second-generation anaplastic lymphoma kinase (ALK) inhibitors. Secondary ALK mutations, ALK gene amplification, and other bypass signal activations (i.e., KRAS mutation, EGFR mutation, amplification of KIT, and increased autophosphorylation of EGFR) are known as resistance mechanisms. However, little has been previously reported on acquired resistance mechanisms to lorlatinib. Here, we report a case of a patient with ALK-positive lung adenocarcinoma that acquired resistance to lorlatinib during treatment for brain metastasis and showed histological transformation to squamous cell carcinoma with MET amplification. We also review the previous literature on the resistance mechanism to ALK inhibitors.
INTRODUCTION
Anaplastic lymphoma kinase (ALK) rearrangements are found in 3%–5% of patients with non-small cell lung cancer (NSCLC).1, 2 Currently, there are four ALK inhibitors approved in Japan by the Pharmaceuticals and Medical Devices Agency, five in Europe approved by the European Medical Agency, and five in the USA approved by the Food and Drug Administration. Based on two phase 3 trials that compared first-line alectinib with crizotinib in ALK-rearranged NSCLC, the standard first treatment for ALK-positive lung adenocarcinoma is alectinib.3, 4 Additionally, the third-generation ALK inhibitor lorlatinib has been shown to be effective in patients with acquired resistance to first-generation or second-generation ALK inhibitors.5
However, there have been few reports on acquired resistance mechanisms to lorlatinib, except for compound mutations in ALK. Here, we report a case of a patient with ALK-positive lung adenocarcinoma that acquired resistance to lorlatinib during treatment for brain metastasis and showed histological transformation to squamous cell carcinoma with MET amplification.
CASE REPORT
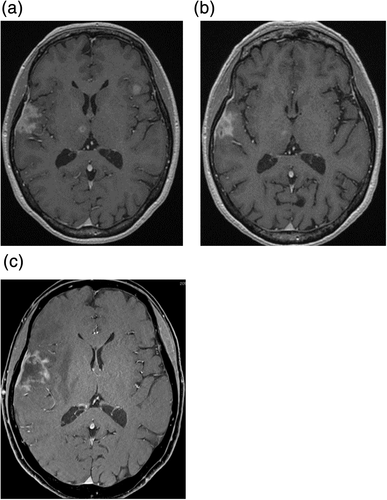
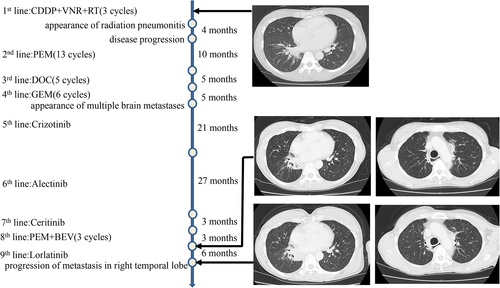
A 58-year-old female non-smoker was diagnosed with clinical T1aN3M0 stage IIIB adenocarcinoma in 2011 (Figure 1(a), (b)). She subseqeuently received concurrent chemoradiotherapy (cisplatin plus vinorelbine with thoracic radiotherapy of 60 Gy in 30 fractions). Computed tomography (CT) showed a good partial response after three cycles of this regimen. However, grade 1 radiation pneumonitis was identified on CT, and further consolidation chemotherapy was eventually discontinued. Her lung cancer lesion and radiation pneumonitis were monitored with CT without any treatment. Four months later, radiation pneumonitis had improved on CT, but progressive disease was identified. She received 13 cycles of pemetrexed, five cycles of docetaxel, and six cycles of gemcitabine. Multiple brain metastases were identified on magnetic resonance imaging (MRI), and the lesions were treated with Gamma knife radiosurgery. Additionally, the test for ALK rearrangement, approved in Japan in 2012, was performed and her lung cancer specimen was found to have ALK rearrangement with fluorescence in situ hybridization (FISH); she subsequently received crizotinib as fifth-line therapy for 21 months. Due to recurrence of thoracic lesions and brain metastases, she was treated with alectinib for 27 months, ceritinib for three months, three cycles of pemetrexed plus bevacizumab, and seven more courses of Gamma knife treatment. However, owing to the recurrence of brain metastases (Figure 2(a)), she received lorlatinib as ninth-line treatment. Four months after initiation of lorlatinib, the brain metastases were under control (Figure 2(b)). During lorlatinib treatment, dose reduction and temporary drug discontinuation were required because of grade 3 edema and grade 2 peripheral sensory neuropathy. Six months after initiation of lorlatinib, she presented at our hospital with hemiparesis. Brain MRI showed an enlargement of the metastatic lesion in the right temporal lobe with severe parenchymal edema (Figure 2(c)). In order to relieve the patient's symptoms, we removed the tumor and the hemiparesis improved. A summary of the treatment course is shown in Figure 3.
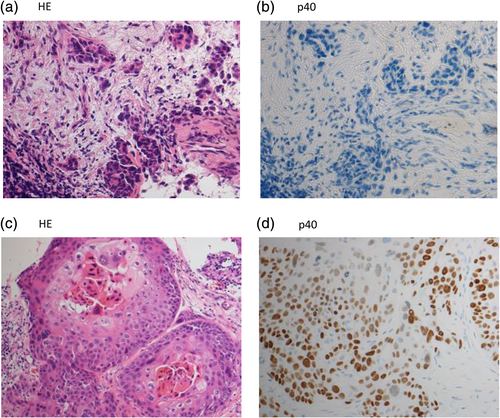
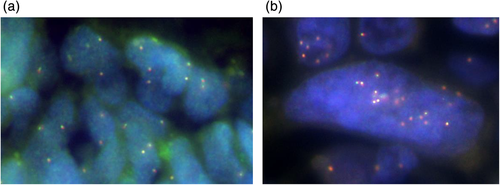
Histopathological review showed the cancer cells in the metastatic brain specimen had changed from adenocarcinoma to squamous cell lung carcinoma (Figure 1(c), (d)). Next-generation sequencing (NGS) of 46 oncogenes was performed with Oncomine Dx Target Test (ThermoFisher Scientific), and it showed no secondary mutations of ALK or other driver oncogenes. Amplification of MET was evaluated with FISH in the samples at the time of diagnosis and after lorlatinib resistance (Figure 4(a), (b)). MET amplification was observed in some cells in the sample at the time of diagnosis, but MET amplification obviously increased in the sample after lorlatinib resistance.
Lorlatinib was resumed after brain tumor resection and continued until CNS progression was confirmed on brain MRI.
DISCUSSION
We report a case of adenocarcinoma that transformed to squamous cell lung carcinoma with MET amplification after resistance to lorlatinib.
There are three generations of ALK inhibitors. Crizotinib is a first-generation ALK inhibitor; ceritinib, alectinib, and brigatinib are second generation inhibitors and lorlatinib is the third generation. Second- and third-generation ALK inhibitors have been developed to overcome resistance to previous generation inhibitors. Secondary ALK mutations are major causes of resistance to inhibitors and were found in 20%–35% of tumors resistant to crizotinib.6-8 ALK gene amplification was also found in 8.3% of crizotinib-resistant tumors, and other bypass signal activations such as KRAS mutation, EGFR mutation, amplification of KIT, and increased autophosphorylation of EGFR were found in tumors resistant to crizotinib.6-8 Secondary ALK mutations were found more frequently in tumors resistant to second-generation ALK inhibitors than in those resistant to first-generation inhibitors.8 Two studies recently reported the mechanism of lorlatinib resistance.9, 10 One study investigated the mechanism of lorlatinib resistance in longitudinal tumor samples from five patients with ALK-positive lung cancer. Similar epithelial-mesenchymal-transition (EMT)-mediated resistance was found in two patients, ALK kinase domain compound mutation-mediated resistance was found in two patients, and NF2 biallelic loss of function mutations were found in one patient.9 Another study showed MET amplification in six (12%) of 52 biopsies following administration of a second-generation ALK inhibitor and in five (22%) of 23 post lorlatinib biopsies. In addition, two tumor specimens harbored an identical ST7-MET rearrangement, one of which had concurrent MET amplification. Dual ALK/MET inhibition resensitized a patient-derived cell line harboring both ST7-MET and MET amplification to ALK inhibitors.10
Histological transformation after first- or second-generation ALK inhibitor treatment has been reported in several studies, wherein most reported on transformation from adenocarcinoma to small-cell lung carcinoma.11 Transformation from adenocarcinoma to squamous cell lung carcinoma was reported after resistance to crizotinib in one case and after resistance to alectinib in another case.12, 13
Our study has some limitations. The tumor specimen after lorlatinib treatment was compared with that taken at the diagnosis of adenocarcinoma. Transformation to squamous cell lung carcinoma with MET amplification may have been acquired as a result of previous treatments before lorlatinib. However, the metastatic lesion in the brain had initially responded to lorlatinib and progressed during lorlatinib treatment. These two changes could be related to lorlatinib resistance. The results of a previous report also suggest that MET amplification could be related to lorlatinib resistance in our case.10 Additionally, lorlatinib is used as a second or further line chemotherapy in clinical practice. It would be difficult to compare tumor samples taken after lorlatinib with those taken just before lorlatinib treatment.
A pooled analysis was conducted to investigate the characteristics and outcomes of 17 patients with EGFR-mutated adenocarcinoma who developed a transformation to squamous cell histology after treatment with EGFR tyrosine kinase inhibitors (EGFR-TKIs).14 Most patients were women (82%), 41% were former smokers, and no current smokers were identified. The median time to squamous cell transformation was 11.5 months. In all cases, basal EGFR mutation was maintained, 11 patients (65%) developed an acquired mutation in exon 20, and a T790M mutation appeared in eight patients (47%). The median survival after squamous cell carcinoma diagnosis was 3.5 months. In the case reported here, there was no smoking history, basal ALK translocation was maintained, and MET amplification was found in addition to squamous cell transformation. Progression-free survival of patients treated with crizotinib has been previously reported to be significantly shorter in squamous cell lung carcinoma with ALK rearrangement than in adenocarcinoma with ALK rearrangement.15 This suggests that squamous cell histology can be related to resistance to ALK inhibitors. The mechanism of resistance to ALK inhibitors, especially lorlatinib, might be multiple and complex. In our case, squamous cell transformation and MET amplification were found in the same patient after treatment with lorlatinib. In this case, it may be difficult to overcome resistance with a molecularly targeted agent aiming to inhibit just one molecule. As reported in the phase 3 CROWN trial which compared lorlatinib with crizotinib, and supports lorlatinib as a future first-line standard treatment in ALK-positive non-small cell lung cancer, knowing the mechanism of resistance to lorlatinib is becoming more important.16 Further investigation is needed to overcome resistance to ALK inhibitors in patients with ALK-positive NSCLC.
ACKNOWLEDGMENT
We are grateful to Kengo Takeuchi, Ryohei Katayama at the Cancer Chemotherapy Center, Japanese Foundation for Cancer Research, Tokyo, Japan for reviewing this manuscript and providing helpful advice.




