Advances in study of the sequence of lung tumor biopsy and thermal ablation
Abstract
Percutaneous thermal ablation is an important treatment for lung cancer and is widely used in hospitals. Puncture biopsy is generally required for pathological diagnosis before or after thermal ablation. Pathological diagnosis provides both evidence of benign and malignant lesions for ablation therapy and is of important significance for the next step in disease management. Furthermore, the sequence of ablation and biopsy affects the accuracy of pathological diagnosis, the complete ablation rate of thermal ablation, and incidence of surgery-related complications. Ultimately, it may affect the patient's benefit from local treatment. This article reviews the research progress of traditional asynchronous biopsy followed by ablation, the emerging methods of synchronous biopsy followed by ablation, and synchronous ablation followed by biopsy in the last decade.
Key points
The sequence of ablation and biopsy affects the accuracy of pathological diagnosis, the complete ablation rate of thermal ablation, and the incidence of surgical-related complications.
This article reviewed the recent 10 years' literature on the surgical sequence of biopsy and ablation for lung tumors, the advantages, disadvantages and indications of different orders were analyzed.
Introduction
Lung cancer is a malignancy with the highest morbidity and mortality among all cancers1, 2 and its incidence has been increasing every year. Currently, the standard treatment for lung cancer is surgical resection, but some patients are unable to undergo surgery due to the advanced stage of disease at the time of diagnosis. Some patients are unable to tolerate surgery because of underlying diseases or advanced age, and some patients are unwilling to undergo surgery. In a study by Ye et al. only 20% of patients were found to meet the surgical indications.3 Thermal ablation has become an important treatment for those patients whose conditions are deemed inoperable. Compared with surgery, ablation has the advantages of being minimally invasive, less expensive, simple, and repeatable. Moreover, the release of antigens after ablation may, to a certain extent, enhance the antitumor immunity of the body.4, 5 The safety and effectiveness of thermal ablation for lung tumors has been confirmed in several studies and is widely recognized.6-14 Clinically, a puncture biopsy is usually required for pathological diagnosis before, or after, thermal ablation. Pathological diagnosis provides evidence of benign and malignant lesions that require treatment. Moreover, distinguishing between the types of lung cancer and gene mutation status has important significance for the next step in disease management. The traditional sequence is selective ablation after pathological confirmation from a puncture biopsy. Over the past decade, some scholars have suggested that synchronous biopsy should be followed by ablation15-17 and some that synchronous ablation should be followed by biopsy,18-24 taking into account factors such as surgical risks, benefits, and actual clinical needs (Table 1). The sequence of ablation and biopsy may have a great impact on the safety and effectiveness of these procedures, although not it has not received adequate attention in clinical practice. This article reviewed the recent 10 years' literature on the surgical sequence of biopsy and ablation for lung tumors, aiming to improve the understanding about the importance of sequence of ablation and biopsy in clinical work and to promote the research and development of minimally invasive treatments for lung tumors.
| Safety | Hemopneumothorax rate was 4.3%; Pneumothorax rate was 7.4%; | Hemorrhage rate was 24%; Pneumothorax rate was 33% | Pneumothorax rate was 29.6%; Hemoptysis and pleural effusion was 22.2% |
|---|---|---|---|
| The one-, two-, and three-year survival rates were 91.3%, 69.6%, and 60.9%, respectively | Local tumor control was achieved in 77% with a median follow-up of 12 months | The effective rate was 100%. | |
| Effectiveness | The positive rate of biopsy was 81.5% | The positive rate of biopsy was 97% | The positive rate of biopsy was 95% |
| Treatment | Synchronous biopsy followed by MWA | Synchronous biopsy followed by RFA | Synchronous biopsy followed by MWA |
| Patient | 23 patients (nine primary lung cancer, 14 metastases) | 28 patients with highly suspicious malignant pulmonary nodules | 21 patients with highly suspicious malignant pulmonary nodules |
| Study type | Retrospective analysis | Retrospective analysis | Retrospective analysis |
| Reference | Liu et al. 21 | Schneider et al.22 | Wang et al.25 |
| Safety | Hemopneumothorax rate was 66.7% | Hemorrhage rate was 5%; pneumothorax rate was 60% | Hemorrhage rate was 32.4%; pneumothorax rate was 60.8% |
| Local tumor control was achieved in 66.6% with a median follow-up of 21 months | Local tumor control was achieved in 95% with a median follow-up of 24 months | ||
| Effectiveness | The positive rate of biopsy was 100% | The positive rate of biopsy was 90% | The positive rate of biopsy was 90.5% |
| Treatment | Synchronous RFA followed by biopsy | Synchronous RFA followed by biopsy | Synchronous MWA followed by biopsy |
| Patient | Three patients (one primary lung cancer, two metastases) | 20 patients with lung nodules were considered malignant | 74 patients with 74 lung GGOs |
| Study type | Case series | Retrospective analysis | Prospective study |
| Reference | Hasegawa et al. 20 | Tselikas et al. 26 | Wang et al.27 |
| Safety | Hemorrhage rate was 1.5%; Pneumothorax rate was 14.7% | Hemorrhage rate was 5.3%; Pneumothorax rate was 47.4% | |
| Local tumor control was achieved in 58.8% with a median follow-up of 21 months | Local tumor control was achieved in 84.2% | ||
| Effectiveness | The positive rate of biopsy was 85.3% | The positive rate of biopsy was 78.9%; Both EGFR and KRAS mutations could be analyzed in 74% of the specimens | |
| Treatment | Synchronous MWA followed by biopsy | Synchronous RFA followed by biopsy | |
| Patient | 68 patients (all patients had primary lung cancer, including 55 adenocarcinomas) | 19 patients | |
| Study type | Retrospective analysis | Retrospective analysis | |
| Reference | Wei et al.28 | Hasegawa et al.29 |
Methods
The articles included in this review were mainly based on reviews or publications published in the PUBMED database from 2010 to 2020, since pulmonary tumor ablation was widely used in clinical practice during this period. This review focuses on the effects of the biopsy and ablation sequence on their effectiveness and safety because different sequences not only affect the accuracy of pathological diagnosis and the complete ablation rate of thermal ablation, but also affect the incidence of surgery-related complications. Ultimately, it may affect the patient's benefit from local treatment, even in the next step of disease management. This review included articles through a keyword retrieval using the terms “pulmonary tumor,” “percutaneous needle biopsy,” “thermal ablation,” “safety,” and “effectiveness.” The articles were divided into three categories: asynchronous biopsy followed by ablation, synchronous biopsy followed by ablation, and synchronous ablation followed by biopsy. Articles with gaps in scientific methods and those without clinical significance were excluded.
Discussion
Asynchronous biopsy followed by ablation
The effectiveness and safety of computer tomography (CT)-guided percutaneous transthoracic needle biopsy (PTNB) and CT-guided thermal ablation of lung tumors as independent procedures have been recognized by clinical consensus, and the treatments are widely used.15, 16, 18, 19, 25-28, 30 Hu et al.30 reported that the coincidence rate of pathological results between small specimens obtained from PTNB of non-small cell lung cancer (NSCLC) and large specimens obtained from surgery was 92.6% (188/203). There was no significant difference between small and large specimens for the classification and diagnosis of lung adenocarcinoma and squamous cell carcinoma (к = 0.872, P = 0.250). Lesion size was an influencing factor for the accuracy of PTNB diagnosis (P = 0.008). Lu et al.25, 26 reviewed the data of 69 lung cancer patients treated with noncoaxial microwave ablation (MWA) and 329 lung cancer patients treated with noncoaxial radiofrequency ablation (RFA). The one- and two-year survival rates were 66.7% and 44.9% (MWA), and 68.2% and 35.3% (RFA), respectively. The main complications of PTNB and thermal ablation of lung tumors are reported to be pneumothorax and pulmonary hemorrhage.15, 16, 18, 19, 27, 28 The incidence of pneumothorax induced by routine biopsy is reported to be 2.4%–60%. Approximately 5%–18% of patients with pneumothorax require an indwelling chest tube, and the incidence of pulmonary hemorrhage is between 5% and 16.9%.28 Moreover, the incidence of pneumothorax induced by ablation is 5%–63%, and the incidence of hemorrhage is 0%–11%.27 With regard to whether pneumothorax is induced by biopsy or ablation, the incidence of pneumothorax reported in the literature varies because of the considerable influence of age, pulmonary basic disease (such as emphysema), and target lesion location of enrolled patients in different studies. However, the overall incidence of pulmonary hemorrhage caused by thermal ablation is lower than that of biopsy, which may be related to the microthrombosis of peripheral vessels caused by thermal ablation.19
Traditionally, the pathology is defined before performing selective ablation, which is in line with the general clinical diagnosis and treatment process. Confirming the pathological results first can avoid unnecessary treatment to a certain extent.27 If physicians have pathological results as the basis for treatment, the ablation goals and endpoints can be better determined. However, the asynchronous method increases the duration of hospital stay and cost. Patients who undergo thermal ablation often have low respiratory function or severe underlying diseases and other high-risk factors. Performing biopsy and thermal ablation separately will expose the patient to two high-risk operations. Biopsy alone may increase the risk of hemorrhage, gas embolism (which may lead to death), and increase patient discomfort.29, 31
Synchronous core-needle biopsy and thermal ablation
Regardless of the method used in synchronous surgery, coaxial cutting biopsy and ablation are recommended. First, the coaxial guide needle can not only reduce the offset caused by the cutting needle's weight and improve the puncture accuracy, but also eliminate the obstruction of the chest wall to the cutting needle to improve the quality of sampling. Second, under the guidance and positioning of the guide needle, not only can the cutting needle angle for multiple sampling be adjusted within a small range, the ablation needle angle for multiple ablations can also be adjusted for in a small range. By reducing the number of pleural punctures, the probability of damage to small pulmonary blood vessels and bronchial branches around the lesion is reduced, thereby greatly reducing puncture complications.
Synchronous biopsy followed by ablation
To reduce the risk of surgery and reduce the length of hospital stay and cost, some scholars have proposed a surgical scheme of simultaneous thermal ablation after percutaneous puncture biopsy of pulmonary lesions and demonstrated its effectiveness and safety.15-17 Liu et al.15 retrospectively analyzed the data of synchronous MWA treatment after CT-guided percutaneous lung biopsy in 23 patients with pulmonary malignant tumors confirmed by preoperative pathology. The results showed that the one, two, and three year survival rates were 91.3%, 69.6%, and 60.9%, respectively, which were higher than those reported in the literature for asynchronous treatment. The positive rate of pathological diagnosis was 81.5%, which was slightly lower than that of PTNB alone. We consider that this could be because only a single specimen was obtained from each patient. However, Schneider et al.16 drew different conclusions by reviewing data from 28 patients with solitary lung tumors that were radiologically suspected of malignancy but could not be treated surgically due to various high-risk factors. RFA was performed immediately after the patient's biopsy specimen was confirmed to be malignant by intraoperative frozen pathology. At an average follow-up of 12 months, although the incidence of various complications in this study was similar to that reported in the literature, the local tumor control rate was only 77%. Therefore, Schneider et al. reported that RFA immediately after CT-guided biopsy is a safe procedure for isolated malignant pulmonary lesions, but considering the local control rate, this method is only suitable for patients who cannot tolerate or refuse surgery. The authors believe that the selected patients in this experiment are all high-risk patients with serious underlying diseases, which to some extent affects the effectiveness of the operation. Notably, the complication rates in high-risk patients are similar to those previously reported in the literature, perhaps demonstrating a higher safety of synchronous biopsy followed by ablation. In terms of safety, Wang et al.[17] retrospectively analyzed MWA data of 62 radiologically highly suspected malignant nodules in 54 patients. Patients were divided into two groups: a synchronous biopsy followed by ablation group and an asynchronous biopsy followed by ablation group. The results showed that the two groups had the same treatment effectiveness, but the incidence of pneumothorax was 29.6% (8/27) in the synchronous biopsy followed by ablation group and 57.1% (20/35) in the asynchronous biopsy followed by ablation group, with a statistically significant difference (P = 0.031). Although the incidence of pulmonary hemorrhage was similar in the two groups, in order to reduce the risk of hemorrhage, the scheme of synchronous biopsy followed by ablation was mostly adopted for patients at a high risk of hemorrhage; thus, the objective selection bias may have affected the final experimental results. For imaging highly suspicious malignant lesions, in order to reduce the operation time and risk, some scholars did not wait for rapid pathological results during the operation and adopted immediate ablation after biopsy. Wang et al. 17 argued that although this method may lead to “overtreatment” of benign lesions, due to the possibility of false-negative results, a negative biopsy cannot completely rule out the possibility of malignancy and that synchronous ablation not only inactivates potential malignant cells but also alleviates patient anxiety, which is still of positive significance to the patient.
Synchronous biopsy followed by ablation reduces the number of surgical procedures, which can not only reduce a patient's hospital stay, cost, and the incidence of complications such as pneumothorax, but RFA and MWA may play a role in the thermocoagulation of hemorrhage induced by biopsy. However, in the same process, pneumothorax and hemorrhage induced by biopsy may affect the efficacy of subsequent ablation, especially for ground-glass opacities (GGOs).20
Synchronous ablation followed by biopsy
To overcome the potential impact of complications such as pneumorrhagia and pneumothorax induced by puncture biopsy during synchronous surgery on the effectiveness of subsequent ablation, some scholars have proposed a surgical scheme for synchronous ablation followed by biopsy.18-24 For the surgical protocol of synchronous ablation followed by biopsy, the main clinical question is whether the pathological diagnosis will be affected by ablation. Some authors have reported that cell apoptosis was gradual after high-temperature treatment.32, 33 In fact, analysis of surgical specimens obtained shortly after the RFA showed that there was residual tumor tissue in the ablated lesions.34, 35 Tselikas et al.20 performed synchronous post-RFA biopsy in 20 patients diagnosed with malignancy by a multidisciplinary oncology committee and qualified for RFA according to the medical history and imaging findings of the tumor before surgery. The results showed that 90% (18/20) of the biopsies could help diagnose malignant tumors, and 70% (14/20) of the tumors were identified as tumor subtypes and sources, including 12 metastatic and two primary lung cancers. The study by Tselikas et al. demonstrated that biopsy after RFA still had a high positive rate of pathological diagnosis. However, in recent years, the requirement for pathological diagnosis has not been limited to identifying benign or malignant tumors and subtypes, especially for primary lung cancer, and gene mutations have played a crucial role in determining the treatment plan.36, 37 In terms of the validity of genetic testing, Hasegawa et al.24 retrospectively analyzed the data of 19 patients who underwent biopsy after RFA. The positive rate of pathological diagnosis was 79% (15/19), and the success rate of genetic testing was 74% (14/19). Five of the six patients (83%) with lung metastases (three with primary lung adenocarcinoma and three with primary colorectal cancer) had the same gene mutations detected in the biopsy specimens after RFA treatment and in the primary resection specimens. Therefore, Hasegawa et al. reported that, even after complete ablation, pathological diagnosis and genetic testing can be performed on biopsy-obtained specimens. At the same time, Hasegawa et al. also pointed out that the antigens used for immunohistochemistry in the specimens obtained after RFA may not be degraded, but the staining may become weakened. Hence, the negative results of immunohistochemistry should be interpreted with caution. These two studies also demonstrated the effectiveness of the surgical method of synchronous ablation followed by biopsy in the treatment of lung cancer. The results of the study of Tselikas et al. showed that during the median follow-up period of 24 months, the local tumor progression rate was 5%, and the 12-month overall survival rate, disease-free survival rate, and progression-free survival rate were 100%, 75%, and 65%, respectively.20 Hasegawa et al.24 reported that the local tumor progression rate over a median follow-up time of 22 months was 16%. The local progression rate reported in previous RFA studies ranged from 12%–32%,38-40 which was slightly higher than the two studies mentioned above.
Both techniques have advantages and disadvantages. Synchronous ablation followed by biopsy may fail to obtain adequate tissue for subsequent biopsy, which affects the accuracy of diagnosis. Therefore, its indications should be carefully considered. At present, most researchers agree that this technique should be used in blood-rich tumors to reduce the occurrence of pulmonary hemorrhage.18, 24 Li et al.19 considered that peripheral lesions (often without surrounding small blood vessels) should be biopsied first and then ablated. Conversely, central lesions (with surrounding small blood vessels and bronchi) should be ablated first in order to prevent asphyxiating hemoptysis caused by cutting the small blood vessels and bronchi simultaneously. Ablation can cause microthrombosis in peripheral vessels and occlusion of bronchioles, which can increase safety.21, 23
In addition to the surgical scheme of synchronous biopsy followed by ablation, synchronous ablation followed by biopsy also has the advantages of shorter hospital stays, lower costs, and fewer complications. Moreover, it overcomes the potential impact of complications such as pneumorrhagia and pneumothorax induced by puncture biopsy on the effectiveness of subsequent ablation and can further reduce the risk of pulmonary hemorrhage and air embolism.21, 23 Although there is little or no evidence supporting this fact, it is theoretically possible to reduce the risk of needle track implant metastasis from needle biopsy. Studies have demonstrated that biopsy specimens obtained after lesion ablation can not only be used for pathological diagnoses, but also genetic testing.20, 24, 34, 35 Therefore, this surgical method is safe and effective, which should be used preferentially in blood-rich tumors and perivascular lesions. However, synchronous ablation followed by biopsy carries the risk of “overtreatment” for benign lesions. Therefore, we should select highly suspicious malignant lesions according to the patient's medical history and imaging manifestations as target lesions and avoid an unclear pathological diagnosis in the subsequent biopsy.
Sequence of synchronous ablation and biopsy for GGO
In recent years, the detection rate of GGO has increased due to the progression of low-dose CT in lung cancer screening. Lung cancers presenting as GGOs are considered lung adenocarcinomas or preinvasive lesions, such as atypical adenomatous hyperplasia or adenocarcinoma in situ, which are difficult to accurately locate and resect during surgery.23 Studies have shown that GGOs and most mixed density nodules are usually inert in biological behavior, rarely accompanied by lymph node or distant metastases.41, 42 Therefore, ablation is a more suitable treatment method after confirming that there is no mediastinal lymph node metastasis.
At present, it is controversial whether the surgical scheme of synchronous ablation followed by biopsy is suitable for GGOs. Some scholars believe that ablation may cause changes in the shape of GGOs, which will make it difficult to distinguish the shape or edge of GGOs during the subsequent biopsy process, ultimately affecting the accuracy of the biopsy.18, 24, 43, 44 In addition, because of the small size of the lesion, it is more likely to be affected by the serious destruction and carbonization of cells around the ablation electrode, which reduces the quality of the specimen obtained.18, 22 In the surgical scheme of synchronous ablation followed by biopsy, Hasegawa et al. 24 found that all biopsy technical failures occurred in GGO cases. After statistical analysis, they concluded that presence of a solid tumor on radiological imaging was the only criteria that affected the success of the biopsy technique (P = 0.02). Therefore, for patients with GGO tumors, they suggested that the pathological diagnosis should be obtained through another procedure before RFA. However, some scholars believe that ablation can aggregate tumor cells inside GGO, which may increase the positive rate of biopsy.23 Meanwhile, vascular microthrombosis and small airway occlusion caused by thermal ablation can reduce the occurrence of bleeding that can cover the GGO as well as the risk of air embolism.21, 23 In a study by Wang et al., 74 GGOs from 74 patients were biopsied before and after MWA to verify their diagnostic efficacy. The results showed that the positive diagnosis rate of biopsy before MWA was 85.1% (63/74) and that of biopsy immediately after MWA was 74.3% (55/74), but the difference was not significant. However, the comprehensive positive rate was 90.5% (67/74), which was higher than that before MWA.21 Taking the above situations into consideration, Li et al. 23 proposed the use of low-power and short-time ablation to “fix” GGOs before the biopsy; this strategy could reduce the risk of bleeding during biopsy and prevent serious damage to the lesions, which could impede the subsequent pathological diagnosis. After obtaining satisfactory biopsy specimens, radical ablation should be performed (Fig 1).
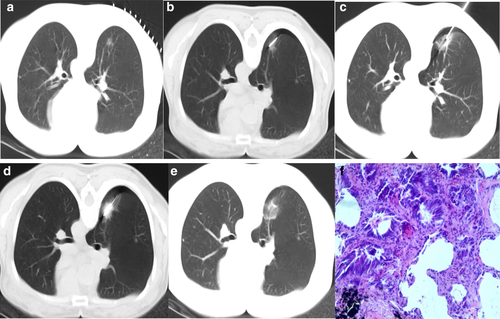
Summary
Lung cancer is a malignant tumor with the highest morbidity and mortality.1, 2 Thermal ablation therapy of lung tumors has become one of the most important alternative treatment methods. With the development of an aging population and the prolongation of life expectancy, the demand for this minimally invasive technique will increase.
Biopsy is an essential procedure both before and after ablation. The sequence of ablation and biopsy may have a great impact on the diagnosis and treatment of lung tumors. Traditionally, the sequence is to first perform a biopsy to confirm the pathological results and then perform selective ablation. Clear biopsy pathological results can help to better determine the ablation goals and endpoints. However, the asynchronous method increases the patient's hospital stay, cost, and surgical risk. The surgical method of synchronous biopsy followed by ablation reduces the number of surgical operations, shortens the length of hospital stay, decreases the cost of treatment, and reduces the risk of pneumothorax and pulmonary hemorrhage. Subsequent ablation also has a certain thermal coagulation effect on the bleeding caused by the biopsy. However, this surgical method has the risk of decreasing the effectiveness of ablation due to the difficulty in tumor targeting caused by the change in image performance after biopsy. Some scholars have further proposed the surgical scheme of synchronous ablation followed by biopsy, which is more conducive to the complete ablation of tumors. Moreover, this surgical scheme can further reduce the risk of pulmonary hemorrhage, air embolism, and needle track implant metastasis caused by biopsy. Current studies have demonstrated that specimens obtained by biopsy after lesion ablation can also achieve pathological diagnoses and genetic testing. However, no studies have investigated whether specimens obtained by biopsy after lesion ablation can be used to detect the predictors of immunotherapy (programmed death receptor 1, tumor mutation burden, and microsatellite instability). The surgical scheme of synchronous ablation followed by biopsy is suitable for highly suspicious malignant lesions, especially those with a high risk of bleeding. However, this surgical scheme has the risk of “overtreatment” for benign lesions, or of making the surgeon passive due to the unclear pathological result of the subsequent biopsy. There is still controversy about which surgical method should be adopted for GGOs.
The sequence of ablation and biopsy is extremely important for the diagnosis and treatment of lung tumors. The safety, efficacy, and indications of various surgical methods still need to be proved by further studies, especially through high-quality prospective randomized controlled trials.
Acknowledgments
This work was supported by the Youth Program of the National Natural Science Foundation of China (No. 81901858), the Graduate Innovation Fund of Peking Union Medical College (No. 2019-1002-88), the Special Fund for Central Health Care of China (No. 2020YB10) and the Capital Health Research and Development of Special (No. 2018-1-4201).
Disclosure
The authors report no conflict of interest.




