Effect of casein genetic variants and glycosylation on bovine milk foaming properties
Abstract
The effects of κ-casein (κ-CN) and β-casein (β-CN) genetic variant and κ-CN glycosylation degree (GD, low or high) on interfacial and foaming properties of bovine skim milk were investigated. No significant effect was measured for milks with different ĸ-CN and β-CN genetic variants. However, milks of higher GD exhibited lower surface tension, enhanced foamability and differences in secondary protein structure compared to lower GD skim milks. Glycan attachment is believed to affect surface activity and the spread and packing of protein at the foam bubble liquid–air interface, leading to differences in foaming performance.
INTRODUCTION
Foaming is defined as a dispersion of air bubbles in a liquid (Walstra 1989) and an important functional property of processed dairy products such as cappuccino-style coffee, whipped cream and ice cream. In these products, foaming properties can influence quality attributes that include appearance, volume, texture and mouthfeel, where consistent foam stability is expected by consumers as products are consumed (Ho et al. 2023). Dairy proteins are commonly used as surfactants in food emulsion and foam systems due to their surface-active properties (Huppertz 2010) and their ability to provide kinetic stability against coalescence. These proteins provide necessary steric and electrostatic repulsion by adsorption at the liquid–air interface to establish a viscoelastic interfacial layer (Ho et al. 2022).
Casein is a heterogeneous mix of four major proteins identified as αs1-casein (αs1-CN), αs2-casein (αs2-CN), β-casein (β-CN) and κ-casein (κ-CN) (Holland and Boland 2014), and accounts for approximately 80% (w/w) of total milk protein. Caseins exist in the form of genetic variants that differ by one or more amino acids that may lead to distinct differences in functionality (Gai et al. 2024; Hewa Nadugala et al. 2024). In addition, the structure of κ-CN differs in nature through the attachment of glycan molecules via the post translational process of glycosylation, influencing stability and functionality (Holland and Boland 2014; Hewa Nadugala et al. 2023).
The presence or absence of specific casein genetic variants in milk has been shown to affect physicochemical and functional properties, recently reviewed by Gai et al. (2021), Daniloski et al. (2022), and Hewa Nadugala et al. (2022). Specifically, the foaming characteristics of milk may be influenced by protein composition and concentration, casein micelle size, pH, temperature, milk fat content, milk fat globule size and mineral balance (Ho et al. 2022). Xiong et al. (2020) performed a study using milk protein concentrate and whey protein concentrate and reported that, while protein concentration had no significant effect on foamability, decreasing the casein-to-whey ratio increased the average air bubble size and substantially decreased foam stability. This was attributed by Xiong et al. (2020) to the lower adsorption and less flexible packing arrangement of the globular whey proteins at the liquid–air interface compared to the intrinsically disordered casein molecules. Furthermore, Lajnaf et al. (2022) reported that β-CN purified from camel milk formed a more stable foam compared to purified bovine β-CN. Camel and bovine β-CN share 84.5% similarities in terms of their amino acid sequence, including residues with similar biochemical properties, indicating that small variations in amino acid sequences may lead to substantial differences in their foaming performance. Therefore, evidence suggests that the adsorption dynamics of proteins at a liquid–air interface during foaming can depend on both their availability, composition and their physicochemical and structural properties.
A few studies have examined the foaming properties of bovine milk relating to the presence of different β-CN genetic variants, however, the results are inconsistent (Ipsen and Otte 2004; Nguyen et al. 2018), possibly due to differences in experimental conditions, such as temperature and foaming method (Ho et al. 2022), where increasing temperature will decrease the apparent viscosity of milk as well as leading to a slight decrease in pH that in turn reduces the protein net charge (Borcherding et al. 2008). For example, Nguyen et al. (2018), found better foam formation in milks consisting of β-CN A2, refrigerated at 4°C, where ambient air was incorporated into the milk using an air pump. Ipsen and Otte (2004), however, discovered better foam formation characteristics with β-CN A1 milk that was foamed at 22°C via mechanical agitation.
To the best of the authors' knowledge, no reported studies have examined the effect of composite β-κ-casein genetic variants on foaming. Moreover, none have focused on the interfacial properties of κ-CN and how differences in structure between the genetic variants could lead to differences in foaming performance, despite the fact that κ-CN protrudes from the outer layer of the casein micelle where it could perceivably influence behaviour at the liquid–air interface. κ-CN glycosylation and its effect on foaming has also not been examined previously, with the exception of one study by Kreuß et al. (2009) who found that foam stabilised with unglycosylated caseinomacropeptides were more stable and rigid compared to foam stabilised with glycosylated caseinomacropeptides, believed to be due to differences in packing of the caseinomacropeptides at the liquid–air interface with and without glycans attached.
It is proposed that the strong negative charge and steric alteration that occurs with glycan attachment effectively modify the structure of the intrinsically disordered structure of κ-CN (Hewa Nadugala et al. 2023), and influence the packing and functional behaviour of the liquid–air interface formed during the aeration and foaming of milk. This builds on the findings of a recent study that examined the influence of glycosylation on the whipping properties of cream (Hewa Nadugala et al. 2024), where the first step of whipping involves the entrapment of large air bubbles stabilised by a thin protein layer at the liquid–air interface. The previous work suggests glycosylation had no effect on overrun during this initial aeration step, but that cream from more highly glycosylated bovine milk creates bubbles with liquid–air protein interfaces that diminish milk fat globule adsorption compared to cream from milk with lower levels of glycosylation, hindering milk fat network formation (Hewa Nadugala et al. 2024). As such, the current study examines foam formation, and the foaming and interfacial properties of bovine milk collected from cows of composite β-κ-CN genetic variants and with either low or high levels of GD.
MATERIALS AND METHODS
Chemicals
Unless otherwise noted, all chemicals were analytical grade or higher grade than analytical grade and bought from Sigma-Aldrich (St. Louis, MO).
Milk sample collection and preparation
The milk from cows of known ĸ-CN and β-CN genetic variants and glycosylation degree (GD) reared in the University of Melbourne Dookie Campus Dairy (Nalinga, Victoria, Australia; latitude 36°41′S, longitude 145°71′E) were collected and verified as described in Hewa Nadugala et al. (2024). Two biological replicates of milk from both the low (L) and high (H) GD categories were collected in October 2022 and January 2023, respectively. Cows within each category were selected on the basis of days-in-milk (DIM), where L and H GD has been shown to associate with early (DIM <100 days) and late (DIM >200 days) DIM, respectively (Hewa Nadugala et al. 2024). Sample collection for experimental purposes was performed with the permission of the Animal Ethics Committee at the University of Melbourne (Ethics ID: 22010). The morning milk from identified cows was collected, refrigerated, transported on ice to the CSIRO Werribee site, and stored overnight at 4 °C before being processed the following day.
Milk samples from cows of the same composite genetic variant and L or H GD group were pooled to form 16 different combinations of milk, containing: ĸ-CN AA, AB and BB, and β-CN A1A1, A1A2 and A2A2; noting that no cows with a ĸ- β-CN composite genotype of ĸ-CN AB β-CN A1A1 or ĸ-CN BB β-CN A1A1 were available within the herd at the time of analysis from the cohort of L GD cows. Each pooled sample was separated into a milk fat and skimmed milk fraction using an Alfa-Laval 106 ae farmhouse cream separator (Alfa-Laval, Melbourne, Victoria, Australia). Residual milk fat remaining in the skim milk fraction was separated via centrifugation (3000 g for 30 min at 4°C) using a Beckman Coulter J6-MI Centrifuge (Gladesville, NSW, Australia) and removed from the surface of the serum phase using vacuum suction. To prevent microbial growth, a preservative (Bronopol, 2-bromo2-nitropropane-1,3-diol; 0.02% (w/w)) was added, and the samples stored at 4 °C. All subsequent analysis were carried out within 1 week of milk collection.
Purified lyophilised samples of unglycosylated ĸ-CN B (UG ĸ-CN B, purity of 74 ± 3%) and glycosylated ĸ-CN B with two glycans (2G ĸ-CN B, purity of 81 ± 2%), were prepared using the method described in Hewa Nadugala et al. (2023) and stored at −20°C prior to analysis for comparative purposes.
Milk compositional analysis and pH
The skimmed milk proximate composition was determined using a LactoScope Fourier transform infrared 20 (Delta Instruments, Drachten, The Netherlands) for % fat, protein and lactose.
Mineral analyses (calcium, potassium, magnesium, sodium and phosphorus) were performed using an inductively coupled plasma optical emission spectrometer (ICP-OES-730, Agilent, Mulgrave, Vic., Australia) as described by Raynes et al. (2018) with minor modifications. In brief, each sample (1 g) was dispersed in concentrated nitric acid (2 mL, 63.6%). The solution was transferred to a microwave tube, and ultra-pure water (1 mL) added before microwave digestion at 1480 W for 40 min with a 20 min ramp, a 20 min hold and cooling. The maximum temperature and pressure were 220°C and 8000 kPa, respectively (Multiwave 5000, Anton Paar GmbH, Graz, Austria). The digested solutions were transferred and diluted to a volume of 30 mL with ultra-pure water. Each sample was measured in triplicate.
The pH of each skim milk was measured at 20°C using a PHM 240 Reference pH Meter (Radiometer, Copenhagen, Denmark) with a 2-point calibration (pH 4 and pH 7), 30 min prior to the foaming experiment.
Casein micelle size
The hydrodynamic diameter of casein micelles within each skim milk sample was measured using dynamic light scattering as described earlier (Hewa Nadugala et al. 2024). The refractive index for the solvent and protein phase was set at 1.33 and 1.45, respectively. Each sample was measured in triplicate.
Fourier transform infrared spectroscopy
A Shimadzu FTIR-8400S spectrometer (Kyoto, Japan) was used to examine protein conformation changes between the skim milk samples. The FTIR spectra of two samples that represent each genetic variants or GD, were collected between 400 and 4000 cm−1, with 80 scans per position and a 4 cm−1 spectral resolution. To avoid large water peaks, samples were lyophilised (Dynavac FD-5, Dynapumps, Melbourne, Vic., Australia) before loading the dried samples into the sample holder and a background spectra scan was performed at the start of the measurements. The experiment was carried out at room temperature (20°C), and the spectra were obtained in absorbance mode. The FTIR data was initially baseline corrected using the Spectragryph software with advanced baseline correction option (v. 1.2.16.1; Oberstdorf, Germany), followed by a derivatisation step (second derivative of the absorbance in the amide I region between 1700 and 1600 cm−1) originally described by Savitzky and Golay (1964). Within the amide I band, several regions were analysed, including the intermolecular/aggregated β-sheet (1700–1682 cm−1); β-turn (1681–1665 cm−1); α-helix (1664–1646 cm−1); random coil (1645–1638 cm−1); intramolecular β-sheet (1637–1615 cm−1); and side chain (1614–1600 cm−1). A curve-fitting procedure was used to estimate the area of each component (in unit of percentage) representing secondary structures of the proteins as described in Mediwaththe et al. (2018). Results were compared with the FTIR analysis of purified UG ĸ-CN B and 2G ĸ-CN B.
Surface tension
Foamability and foam stability
Foam bubble size
Images of the foam surface were captured using a light microscope (Leica DM6000 B; Leica Microsystems Pty Ltd, Macquarie Park, NSW, Australia) equipped with a Leica DFC450 C Digital 5 Megapixel colour camera system (Leica Microsystems Pty Ltd) and illuminated with a Leica EL6000 lamp (Leica Microsystems Pty Ltd). Three images were taken immediately after foaming (t = 0) and after 10 min and analysed for air bubble diameter using Image J software (National Institutes of Health, Wisconsin, USA) according to the method of Ho et al. (2021).
Statistical analysis
All experimental data, with the exception of the FTIR and regression analysis, was evaluated using linear mixed models, fitted using Genstat 22nd edition statistical software (VSN International Ltd.). The fixed effects were ĸ-CN, β-CN, GD and protein concentration. The random effects were the pooling of milk from (up to 3) individual cows per sample combination, and biological replicates nested within these combinations. Regression analyses were performed using Minitab statistical software version 19.2020.1 (Minitab Ltd, Coventry, UK). FTIR results that assess differences in peak areas across multiple spectral peaks was evaluated using one-way analysis of variance and Tukey's Studentised Range post-hoc tests, using Python 3.8 (Python Software Foundation, Wilmington, DE, USA), with Pandas for data handling and SciPy for statistical calculations.
RESULTS AND DISCUSSION
Differences in skim milk compositional parameters with β-CN or κ-CN genetic variant and κ-CN glycosylation degree
Table 1 shows the chemical composition of the skim milk samples, grouped by their κ-CN and β-CN genetic variant and GD. This investigation involved the evaluation of three κ-CN genetic variants, namely AA (n = 12), AB (n = 10) and BB (n = 8), three β-CN genetic variants, namely A1A1 (n = 7), A1A2 (n = 11) and A2A2 (n = 12) and two GD groups, H (n = 17) and L (n = 13). An antimicrobial ingredient was used to prevent microbial growth in the absence of a heat treatment step that was purposefully excluded to mitigate the potential impact of elevated temperatures on protein structure. However, it is important to note that the antimicrobial ingredient would not inactivate enzymes present in the milk, hence enzymatic proteolysis may still have occurred leading to changes to the protein fraction over time.
| Parameter | κ-CN GV | Mean ± SD | β-CN GV | Mean ± SD | GD | Mean |
|---|---|---|---|---|---|---|
| Protein (%) | AA | 3.06 ± 0.23 | A1A1 | 3.24 ± 0.41 | H | 3.46a ± 0.31 |
| AB | 3.18 ± 0.38 | A1A2 | 3.26 ± 0.36 | L | 2.90b ± 0.12 | |
| BB | 3.50 ± 0.39 | A2A2 | 3.16 ± 0.36 | |||
| Fat (%) | AA | 0.15a ± 0.02 | A1A1 | 0.20 ± 0.11 | H | 0.19 ± 0.07 |
| AB | 0.17a ± 0.03 | A1A2 | 0.16 ± 0.04 | L | 0.18 ± 0.04 | |
| BB | 0.24b ± 0.09 | A2A2 | 0.18 ± 0.04 | |||
| Casein micelle size (nm) | AA | 211.02a ± 19.36 | A1A1 | 207.58 ± 23.20 | H | 195.96 ± 26.35 |
| AB | 187.56b ± 14.37 | A1A2 | 187.02 ± 23.50 | L | 182.17 ± 22.22 | |
| BB | 161.46c ± 11.34 | A2A2 | 182.44 ± 24.15 | |||
| Calcium (mg/L) | AA | 1030.38a ± 72.14 | A1A1 | 1084.60 ± 101.32 | H | 1080.46 ± 85.26 |
| AB | 1088.94a ± 60.79 | A1A2 | 1063.80 ± 87.94 | L | 1087.76 ± 93.45 | |
| BB | 1154.83b ± 88.04 | A2A2 | 1100.69 ± 79.74 | |||
| Potassium (mg/L) | AA | 1435.12 ± 85.64 | A1A1 | 1384.72 ± 84.44 | H | 1377.81a ± 68.44 |
| AB | 1445.61 ± 67.55 | A1A2 | 1453.32 ± 74.77 | L | 1500.99b ± 60.81 | |
| BB | 1407.34 ± 113.57 | A2A2 | 1436.87 ± 96.39 | |||
| Magnesium (mg/L) | AA | 95.03 ± 8.88 | A1A1 | 100.34 ± 8.10 | H | 102.04b ± 7.98 |
| AB | 96.03 ± 8.94 | A1A2 | 100.55 ± 10.85 | L | 92.70a ± 9.11 | |
| BB | 104.72 ± 8.48 | A2A2 | 94.44 ± 8.30 | |||
| Sodium (mg/L) | AA | 187.13 ± 44.25 | A1A1 | 202.10 ± 45.17 | H | 207.03b ± 31.08 |
| AB | 176.24 ± 26.63 | A1A2 | 183.69 ± 30.55 | L | 152.14a ± 18.58 | |
| BB | 186.32 ± 39.86 | A2A2 | 172.57 ± 36.35 | |||
| Phosphorus (mg/L) | AA | 716.99a ± 36.91 | A1A1 | 740.65 ± 46.30 | H | 734.82 ± 44.92 |
| AB | 722.94a ± 32.18 | A1A2 | 740.76 ± 53.44 | L | 733.63 ± 47.65 | |
| BB | 773.30b ± 49.73 | A2A2 | 725.04 ± 37.20 |
- Results are the means ± SD. Values within a column for each parameter that do not share a common superscript letter are significantly different (P ≤ 0.05) based on a linear mixed model.
A significant difference was noted in the fat concentration achieved through skimming for milks of the different κ-CN genetic variant groupings, varying from 0.15 to 0.24% (w/w), but not between the β-CN groupings or between H and L GD. While small changes in fat concentration may affect foaming properties (Ho et al. 2020), the difference in fat measured between samples in the current work did not significantly affect foamability or foam stability after either 10 or 20 min (P > 0.05). The pH of the skim milk samples was within the narrow range expected for fresh milk under normal conditions (average pH = 6.6 ± 0.1) (De Marchi et al. 2009; Ho et al. 2022).
Protein concentration
The protein concentration of skim milks was not standardised in the current work and some differences were observed between samples that should be considered when interpreting results. For this purpose, protein concentration was considered a fixed effect in the linear mixed model used to analyse the data, allowing for the individual, combined and interactive effects of protein concentration, genetic variant and GD to be identified.
No significant difference in protein concentration was found for milks of different κ-CN and β-CN genetic variants, though higher protein levels have been found by others in κ-CN BB milks compared to milks with κ-CN AA, while κ-CN AB tends to exhibit an intermediate protein concentration (Čítek et al. 2021; Amalfitano et al. 2022). It is noteworthy that milk characterised by a higher GD exhibited a protein concentration that was roughly 20% greater (3.46% (w/w)) than milk with a lower GD (2.90% (w/w)). A similar trend was previously identified in bovine milk by Li et al. (2022) for the milk of seasonally calved cows in early and late lactation, which has been shown to correlate with low and high GD, respectively (Hewa Nadugala et al. 2024).
Additionally, it is important to note that dairy systems are complex, and many other on-farm and compositional factors not measured could contribute to the observed outcomes discussed herein. This can include factors influencing the make-up and function of the protein fraction that may change as a season progresses, such as casein-to-whey ratio (Walker et al. 2004) and the proportion of αs- or κ-CN to total casein (Ostersen et al. 1997).
Mineral concentration
The concentration of selected minerals found in the skim milk samples are shown in Table 1. Significant differences were measured in levels of calcium and phosphorus between milks of different κ-CN genetic variants, and for potassium, magnesium and sodium with GD. No difference was found between milks of different β-CN genetic variants. Levels of calcium and phosphorus were higher in milks containing κ-CN BB compared to κ-CN AA as shown previously by (Jensen et al. 2012), possibly connected to the higher protein concentration measured in the κ-CN BB milks (Dunshea et al. 2019).
Higher levels of sodium and magnesium were measured in the H GD samples, whereas higher levels of potassium were found in the L GD group. Previous work has suggested that glycan attachment leads to the modification of the protein secondary structure, exposing more negatively charged and hydrophilic sites (Hewa Nadugala et al. 2023). These sites, in combination with the greater number of negatively charged glycans found in the H GD group samples, promote the binding of salts (Brockhausen et al. 2022) and might contribute to the higher levels of sodium and magnesium measured in the H GD group compared to the L GD samples. The greater affinity for sodium binding at protein surfaces compared to potassium (Vrbka et al. 2006) could also possibly explain why the same positive correlation was not observed between potassium and GD, with sites available across the unfolded glycosylated κ-CN preferentially accessed by sodium ions at the expense of potassium. Instead, the hydrated potassium ions, known to be smaller in size than hydrated sodium ions (Vrbka et al. 2006), could more easily access the negatively charged sites hidden within the unfolded, unglycosylated κ-CN, leading to the higher levels of potassium measured for the L GD group.
Casein micelle size
A comparison between the casein micelle size of the skim milk samples composed of different κ-CN and β-CN genetic variants and GD is shown in Table 1. Aligned with observations made earlier for different milks collected to achieve the same milk grouping utilised herein (Hewa Nadugala et al. 2024) and the earlier work of Bijl et al. (2014), the average casein micelle size varied between milks of different κ-CN genetic variants (κ-CN AA > AB > BB). Little difference was found between β-CN genetic variants, consistent with the findings of Day et al. (2015), as well as between the H and L GD groups.
Secondary protein conformation observed using Fourier transform infrared spectroscopy
Table 2 presents the quantification of differences in the protein secondary structure measured in the amide I region (1700–1600 cm−1) of all lyophilised skim milk samples. A representative FTIR spectrum of skim milk containing L and H GD is shown in Figure 1. No significant differences in FTIR spectra, hence secondary protein structure, were observed between κ-CN and β-CN genetic variants. However, the results show differences in secondary structure between samples in the H and L GD groups, including the level of contribution of side chains, intra- and intermolecular β-sheet, random coil, α-helix and β-turn structural elements to the overall secondary structure of the proteins. Importantly, the same pattern was observed between the FTIR spectra of purified unglycosylated κ-CN B and κ-CN B with two glycans attached (Table S1), confirming that the structural changes observed in the skim milk samples of the current study are influenced by glycosylation.
| Wavenumber (cm−1) | Secondary structure | Area (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| κ-CN GV | β-CN GV | κ-CN GD | |||||||
| AA | AB | BB | A1A1 | A1A2 | A2A2 | L | H | ||
| 1700–1686 | Intermolecular β-sheet | 8.38 | 8.25 | 8.25 | 8.38 | 8.26 | 8.26 | 8.64a | 4.72b |
| 1684–1672 | β-turn | 6.35 | 6.26 | 6.26 | 6.35 | 6.26 | 6.26 | 6.33a | 11.30b |
| 1672–1655 | α-helix | 38.23 | 37.68 | 37.67 | 38.22 | 37.67 | 37.68 | 38.12a | 43.63b |
| 1653–1636 | Random coil | 24.81 | 24.46 | 24.46 | 24.82 | 24.45 | 24.45 | 24.75a | 18.75b |
| 1636–1622 | Intramolecular β-sheet | 10.84 | 11.55 | 11.55 | 10.84 | 11.55 | 11.55 | 10.80a | 5.31b |
| 1620–1600 | Side chain | 11.40 | 11.81 | 11.82 | 11.40 | 11.82 | 11.82 | 11.37a | 16.29b |
- Mean values within a row that do not share a common superscript letter are significantly different (P ≤ 0.05) based on one-way analysis of variance.
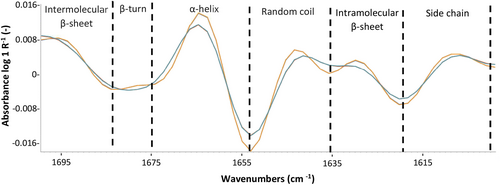
The proportion of inter- and intramolecular β-sheet structures decreased (~10%) with glycan attachment, along with a respective increase in α-helix and β-turn structures (~10%), suggesting that glycan attachment leads to the conversion of protein segments from β-sheets to α-helix and β-turn structures. The surface hydrophilicity of casein negatively correlates with the content of β-sheet structures (Ragab et al. 2020), and positively with the content of α-helixes (Wang et al. 2011), supporting the current understanding whereby H GD samples are more hydrophilic in nature. The current results confirm earlier findings, where shifts in the tryptophan intrinsic fluorescence spectra of milk fractions containing no or two glycans were characterised (Hewa Nadugala et al. 2023).
The effect of β-CN or κ-CN genetic variant and κ-CN glycosylation degree on surface tension
The static and dynamic surface tension of the skim milk samples are shown in Figure 2, providing information on the cohesive forces between molecules at the liquid–air interface, and the effectiveness of dairy proteins as surfactants that can stabilise an interfacial layer, respectively (Tricot 1997; Tamm et al. 2012). Little difference in static surface tension was measured between milks within the different κ-CN or β-CN genetic variant groupings (Figure 2a,b). However, samples in the L GD group exhibited significantly higher static surface tension (46.9 ± 0.8 mN/m) compared to the H GD group (43.1 ± 2.7 mN/m) (Figure 2c).
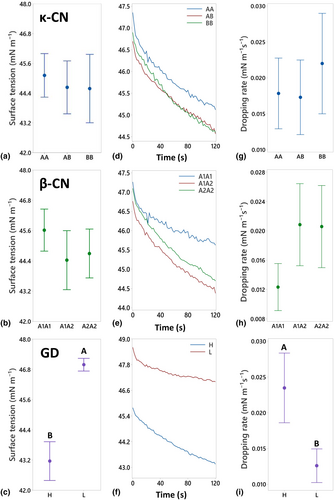
Changes in protein concentration can affect surface tension, particularly in dilute systems at lower concentrations. For example, the surface tension of a sodium caseinate solution rapidly decreased with increasing protein concentration up to around 2.5%, after which the surface tension plateaued (Amine et al. 2014) indicating an overload affect at higher protein concentrations. Though bovine milk is not a diluted system, with average concentrations typically around 3.5% (Dairy Australia 2012), the protein content of the L GD group (2.90%) was lower than the H GD group (3.46%), and potentially within a window where protein concentration may still influence surface tension. As such, linear mixed model statistical analysis was performed to better understand the different contributing factors. The analysis confirmed a strong association between GD (P < 0.001) with surface tension. However, no individual (P = 0.550) or interactive (P = 0.610) contribution was measured for protein concentration, indicating that overall differences measured between the H GD and L GD group samples were not influenced by protein concentration, possibly due to the higher and relatively small difference in protein concentrations between groupings (2.90 ± 0.12 and 3.46 ± 0.31%, respectively). However, a significant effect was noted for protein concentration (P = 0.004) when in combination with GD (P < 0.001), which reflects the individual linear relationships noted between protein concentration and surface tension when samples from within each GD group were plotted separately (Figure S1).
The dynamic surface tension data exhibits a similar pattern to those observed in static surface tension, with the dynamic change in surface tension over time shown in Figure 2(d–f) and the rate of change in surface tension (referred to as the ‘dropping rate’) shown in Figure 2(g–i). Again, little difference was observed in dynamic surface tension between samples within the κ-CN and β-CN genetic variants groupings indicating that the rate of protein adsorption to the liquid–air interface is similar (Wüstneck et al. 1996). However, the dropping rate was significantly higher in H GD samples compared to L GD group, indicating that proteins with higher GD adsorb faster to the liquid–air interface (Zhi et al. 2024).
Combined, the FTIR, static and dynamic surface tension results suggest the difference in conformational folding observed between the L and H GD groups contributes most greatly to this difference in surface tension at the liquid–air interface, over and above any potential effect of protein concentration. The structural modification of glycosylated κ-CN exposes more negatively charged sites and increases hydrophilicity, leading to greater surface activity, that is, the protein's ability to spread at the interface. Thus, an elevated proportion of glycosylated κ-CN in relation to the total κ-CN, as per the H GD group, enhances the overall hydrophilic properties of the skim milk sample. In combination with the unfolded structure of the glycosylated κ-CN, this phenomenon may enhance adsorption rates and the preferential orientation and packing of proteins at the liquid–air interface (McClements 2015). The open structure of glycosylated κ-CN allows for greater spread of the protein at the interface, leading to an increase in surface coverage and a corresponding decrease in surface tension (Wang and Morgner 2014). This is supported by the findings of Xiao et al. (2020) who have shown an inverse association between hydrophilicity and surface tension at protein interfaces.
The effect of β-CN or κ-CN genetic variant and κ-CN glycosylation degree on foamability and foam stability
The foamability and the foam stability of the skim milk samples with different κ-CN and β-CN genetic variants, and GD groups are shown in Figure 3. The foamability of milk relates to its capacity to form a foam, primarily determined by the surface activity of molecules at the liquid–air interface. Foam stability refers to the foams ability to maintain structure and resist collapse over time (Huppertz 2010), governed by interactions between neighbouring air bubbles, and the resilience of the interfacial protein layer (McClements 2009; Ho et al. 2022) that serves as a viscoelastic barrier.
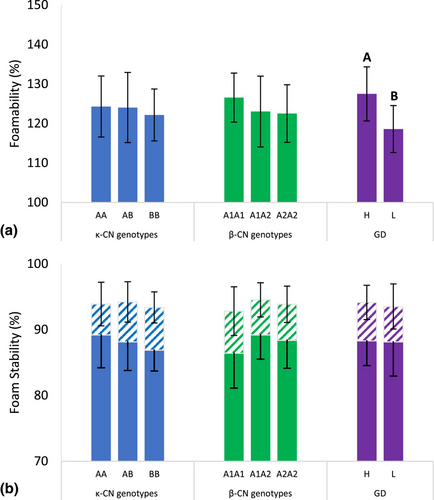
Little difference in foamability was observed between milk groupings of the different κ-CN and β-CN genetic variants. However, a distinct difference was found between the H and L GD groups, with the H GD group exhibiting higher (127.5 ± 6.8%) foamability compared to the L GD group (118.6 ± 6.0%) (Figure 3a). This reflects an average difference of 13.35 mL in foam volume between the two groupings, which is visible to the naked eye and would affect the consumer experience of products where foam is a mark of quality. A linear mixed model was again performed to explore whether this difference was moderated by the difference in protein concentration measured between the H GD and L GD groups (H GD > L GD). The analysis confirmed strong associations between protein concentration (P = 0.009) and GD (P = 0.002) with foamability, but that there was no significant interaction between the two factors on foamability (P = 0.863). This indicates that differences measured between the H GD and L GD group samples were influenced separately by the effect of both individual factors, however noting that, when both factors were taken in combination, GD (P = 0.077) was shown to have more influence than protein concentration (P = 0.482). This is likely the result of the increased surface activity of the glycosylated κ-CN shown earlier (Figure 2), with greater coverage of the interfacial liquid–air protein layer compared to the unglycosylated κ-CN, having a positive effect on the volume of foam produced.
However, the stability of foams prepared from skim milk samples of the H and L GD groups were similar after standing for 10 and 20 min indicating that the volume of foam present for each sample decreased at a similar and proportionate rate with time. Similarly, little difference in foam stability was observed between the samples of differing κ-CN and β-CN genetic variants (Figure 3b). These findings indicate that the effect of κ-CN glycosylation did not affect foam destabilisation processes such as gravitational drainage, that is, where air bubbles rise to the top and separate from the denser liquid phase, air bubble coalescence (Huppertz 2010) and Ostwald ripening (Fredrick et al. 2010).
The effect of β-CN or κ-CN genetic variant and κ-CN glycosylation degree on foam air bubble diameter
Air bubble diameter of freshly prepared foam
Representative images and analysis for bubble size of freshly prepared foams at time zero from skim milk samples with different κ-CN and β-CN genetic variants and GD groups are shown in Figure 4. The mean diameter of bubbles of the freshly prepared foams did not differ between the samples of different κ-CN and β-CN genetic variants, however a significant difference was observed between the H and L GD groups. While some larger bubbles were observed in the foam prepared from the H GD skim milk, a greater number of larger bubbles were evident in the L GD foam, and the mean bubble size was larger (66.2 μm) compared to the H GD group (56.2 μm).
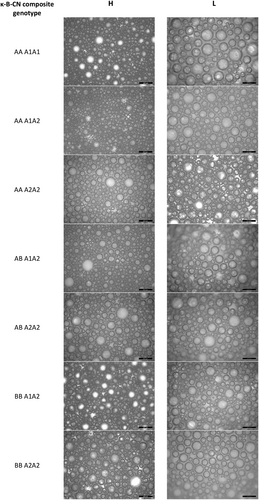
Linear mixed model analysis was again used to determine if this affect was moderated by differences in protein concentration. The analysis confirmed individual associations between protein concentration (P = 0.011) and GD (P = 0.021) with bubble diameter, but there was no significant interaction between either factor on bubble size (P = 0.668). This indicates that the diameter difference measured between the H GD and L GD group samples were influenced separately by the effect of both individual factors, however in this case, the protein concentration (P = 0.212) was shown to have more influence than GD (P = 0.493) when taken in combination. As such, the higher protein concentration of the H GD skim milk samples provided the materials needed to form smaller bubbles with a greater surface area. Previously, Xiong et al. (2020) has shown an increase in protein concentration facilitates the generation of smaller air bubbles, leading to an increase in overall smoothness of the foam texture. At the same time, the higher surface activity and resistance to interfacial rupture proposed previously for H GD group samples (Hewa Nadugala et al. 2024) hindered the coalescence of smaller bubbles into larger ones as the skim milk was agitated to form the initial foam.
Changes in foam air bubble diameter with time
No difference in mean air bubble diameter was found between foams prepared using the skim milk samples of different κ-CN and β-CN genetic variants and measured at the 10 min point (data not shown).
A comparison of air bubble diameter for the freshly prepared H and L GD group foams and after 10 min of standing are shown in Figure 5. A significant increase in mean air bubble diameter was measured for foams from the H GD group with time, shifting from 56.2 to 63.7 μm. In contrast, no significant change in mean bubble diameter was observed for the freshly prepared L GD group foam (66.2 μm) compared to 10 min of standing (66.8 μm). As such, differences observed in bubble size for the freshly prepared H and L GD group foams did not persist with time, and coalescence or Ostwald ripening occurred more readily for H GD group samples.
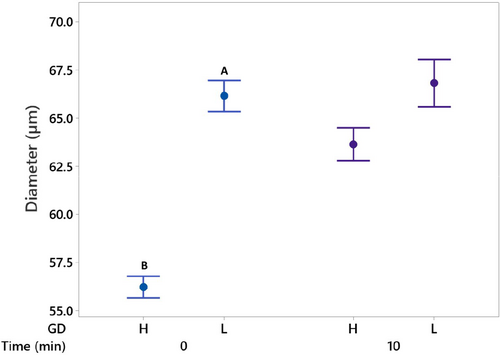
The rate of coalescence can be influenced by collision frequency (McClements 2009), which would be higher in the H GD foam samples where the initial number of smaller bubbles per unit volume was higher compared to the L GD group. However, the increased resistance to rupture suggested in previous work (Hewa Nadugala et al. 2024), supported by the difference in air bubble diameter observed herein for the freshly prepared foams and possible higher surface loading of protein due to lower surface area of smaller air bubbles in H GD milks, suggests that coalescence is less likely to have occurred and that the change in bubble size was probably the result of Oswald ripening; though noting that further work is needed to confirm this hypothesis. Smaller bubbles exhibit a greater internal pressure compared to larger bubbles (Huppertz 2010), and the pressure gradient between the smaller air bubbles and the environment facilitates air molecule diffusion, enabling smaller bubbles to dissolve and larger bubbles to concurrently expand as Ostwald ripening continues (McClements 2009). This proposed mechanism supports the observed change in mean bubble diameter for the H GD group samples. In addition, the rate of Ostwald ripening can be influenced by the nature of the interfacial layer where the greater surface tension exhibited by the L GD group samples may have hindered air molecule diffusion and prevent Ostwald ripening from occurring, leading to little change in air bubble diameter with time for the L GD group.
CONCLUSIONS
Smaller casein micelles were measured in skim milks containing κ-CN BB compared to the κ-CN AA and AB genetic variants, confirming earlier results. However, no effect of micelle size on foaming and interfacial properties were observed. Moreover, no significant association was found between foamability or foam stability between skim milks of the differing ĸ-CN genetic variants (AA, AB, BB) or β-CN genetic variants (A1A1, A1A2, A2A2), indicating that the small conformation changes in amino acid structure between casein genetic variants do not influence the foaming performance of bovine milk. Indeed, little difference in protein secondary structure was measured between skim milks of the differing ĸ-CN genetic variants using Fourier Transform Infrared spectroscopy, whereas significant differences were noted for levels of all structural elements quantified in the amide I region between higher and lower GD skim milk, confirmed through the FTIR analysis of purified glycosylated and unglycosylated ĸ-CN B.
Protein concentration has an overarching effect on skim milk foamability, demonstrated earlier by others and evidenced through the current work with significant associations determined for protein concentration with foamability and bubble size. However, over and above this effect, skim milk with a higher GD was shown for the first time to form air bubbles with lower surface tension and to exhibit enhanced foamability compared to skim milk with a lower GD. This indicates that highly glycosylated skim milk performs more favourably during foam formation, likely due to the greater adsorption and possible greater spread and packing of the glycosylated ĸ-CN at the liquid–air interface of air bubbles formed during foam formation. Moreover, a greater volume of foam consisting of smaller air bubbles formed using the higher GD skim milk compared to milk with a lower GD, which may enhance the quality and consumer experience of freshly prepared cappuccino-style beverages. Measures for foam stability indicate little difference in the rate of foam destabilisation with GD, whereby skim milk of a higher GD would exhibit a greater volume of foam at any given time point. However, the smaller bubbles of the freshly prepared foam increased in size, likely due to Ostwald ripening, and the bubble size distribution of the higher and lower GD milks were similar after 10 min of standing. These results could assist the dairy industry in selecting milks from cows with higher levels of glycosylation for optimum foaming performance.
ACKNOWLEDGEMENTS
The authors would like to thank Mr. Louie Minoza and his team (The University of Melbourne Dookie dairy farm), for sample collection, Dr Roderick Williams, Ms Toni Harrison, Dr Thu McCann, Dr Mya Myintzu Hlaing, Ms Allison Williams and Dr Simon Loveday (CSIRO), for their help with laboratory experiments and/or scientific discussion, and Mr Michael Mazzonetto (CSIRO) for assistance with sample preparation. Authors would also like to thank Dr Tian Zheng (The University of Melbourne) for performing the surface tension analysis as part of the Materials Characterisation and Fabrication Platform, and Dr Christian Davey (The University of Melbourne) for help with statistical analysis as part of the Statistical Consulting Centre. The authors would like to acknowledge CSIRO's ‘Active Integrated Matter’ (AIM) Future Science Platform, Sri Lanka Science and Technology Human Resources Development (STHRD) Project of the Asian Development Bank, and the University of Melbourne's Dr Albert Shimmins fund and Research Scholarship for supporting this project.
AUTHOR CONTRIBUTIONS
Barana Hewa Nadugala: Conceptualization; methodology; investigation; writing – original draft; visualization. Graham Hepworth: Methodology; formal analysis; writing – review and editing. Nuwan R Vithanage: Formal analysis; writing – review and editing. Charles N Pagel: Conceptualization; supervision; resources; writing – review and editing. Jared K Raynes: Supervision; writing – review and editing. C Senaka Ranadheera: Conceptualization; supervision; resources; writing – review and editing. Amy Logan: Conceptualization; methodology; formal analysis; supervision; resources; writing – review and editing.
CONFLICT OF INTEREST
Authors declare that they do not have a conflict of interest in publishing this work, including competing or financial.
Open Research
DATA AVAILABILITY STATEMENT
Data will be made available on request.




