Oxidation and degradation kinetics of lactic butter supplemented with Myrtus communis essential oils
Abstract
The aim of this study was to investigate lipid oxidation/degradation kinetics of lactic butter manufactured from heat-treated and cultured cream, supplemented with Myrtus communis essential oils and stored at 4°C. Lipid oxidation followed zero-order, while vitamin degradation followed first-order reaction kinetics in fresh lactic butter. Optimising heat treatments of the cream at 70°C and 80°C for 30 s and using M. communis EOs reduced lipid oxidation and α-tocopherol degradation (P ≤ 0.05) of lactic butter samples. It was concluded that M. communis EOs is effective as antioxidant as compared to synthetic ascorbyl palmitate for improving stability of lactic butter.
INTRODUCTION
Butter is an important dairy product in human nutrition, it melts at body temperature and is easily digested (Méndez-Cid et al. 2017; Cakmakci et al. 2023). For enhanced flavour, butter can be prepared from cultured and ripened cream. Lactic butter (LB), made from dairy cream and fermented with LAB starter culture, has a sour and aromatic taste and a longer shelf life than conventional butter (Aryana and Olson 2017; Seo 2023). It is a popular soured Turkish dairy product. Cream processing and storage conditions significantly affect the oxidative stability of butter (Khan et al. 2019; Basheer and Muthusamy 2022). The peroxide value (PV) is used to determine oxidation status, while thiobarbituric acid reactive substances (TBARs) is a useful parameter to estimate secondary oxidation products in dairy products (Khan et al. 2019). The shelf life of butter is important, and consumers want butter with high nutritional value and improved oxidative stability (Park et al. 2014; Boulares et al. 2023). Essential oils in M. communis has powerful biological activities as an antioxidant (Aleksic and Knezevic 2014; Hennia et al. 2019; Snoussi et al. 2021; Al-Maharik et al. 2023). To mitigate undesired chemical reactions, it is imperative to carefully select optimal heating temperatures and times (Toledo et al. 2018). Oxidation and degradation reactions occur with low activation energy, resulting in flavour defects, colour and quality loss, nutritional value reduction and consumer rejection (Méndez-Cid et al. 2017; Basheer and Muthusamy 2022; Boulares et al. 2023). To the best of our knowledge, this is the first study to demonstrate the effect of both heat treatments of cream and use of essential oil M. communis on oxidation and degradation of LB. The aim of this study was to investigate the kinetics of lipid oxidation and vitamin degradation of the lactic butter made from heat-treated (70°C, 80°C and 90°C for 15, 30 and 60 s) and cultured cream, supplemented with M. communis EOs and AP (at 200 ppm) and stored at 4°C. The data were analysed using a linear Arrhenius model to find appropriate kinetic models and statistical methods to investigate the relationship between heating temperature, time, antioxidants, lipid oxidation and vitamin degradation in lactic butter during production and storage.
MATERIALS AND METHODS
Materials
The purity of all standards was stated to be approximately 98%. The chemicals and solvents used in this study were of HPLC grade and were obtained from Merck (Darmstadt, Germany). Pure water were used in the analyses (New Human Power, South Korea). Ascorbyl palmitate (L. ascorbic acid-6-palmitate) was obtained from Sigma-Aldrich (Steinheim, Germany), and M. communis L. essential oil was obtained from ABT Normal Products (Istanbul, Turkey). The polypropylene oval butter bowl used as the packaging material was obtained from Zirve Packaging (Adana, Turkey).
Methods
Manufacturing of lactic butter
Fresh cow's milk cream (standardised to 40 % fat, pH 6.6, 135 kg) was batch heated at 70°C, 80°C and 90°C for 15, 30 and 60 s and then cooled to 45°C. The creams were inoculated with 2% yogurt culture (YO-MIX TM 511 LYO 100 DCU, Danisco, Denmark). The cultured cream was ripened for 3–4 h at 45°C until the pH dropped to 5, and then the creams were transferred to a cold room and left at 4 ± 1°C for 48 h. The production flow chart of the butter is shown in Figure 1.
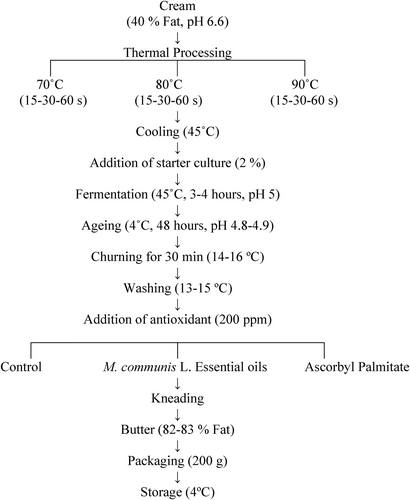
The ripened cream (pH 4.8–4.9) was churned using a churn (Minisan, Turkey) into lactic butter at 14–16°C for 30 min. The churning was conducted in churns filled to 40% with cream. Buttermilk was separated, and the butter samples were washed with cold water (13–15°C), and excess water was removed by draining. The LB samples were then mixed and divided into three batches. Preliminary sensory and chemical analysis results showed that the most appropriate concentration (0–1000 ppm) for M. communis EOs was 200 ppm. This study was carried out using the antioxidants at levels of 200 ppm. Thus, the first group served as the control, and M. communis EOs (200 ppm) and AP (200 ppm) were added to the second and third groups and mixed well in an electric mixer (Osimo, Turkey). The LB samples were then packed in polypropylene oval butter bowl (200 g) and stored in the dark at 4°C for 50 days, with analyses performed at 10-day intervals. The production of butter was repeated in duplicate; total 70 kg butter samples (minimum 82% fat with 51.8% production efficiency) were produced in each trial. For each trial, the same procedure was used (total of 324 × 2 samples), and the experimental design was set up as follows: 3 [heating temperatures (70, 80, 90°C)] × 3 [heating times (15, 30, 60 s)] × 3 [antioxidant (Control, M. communis EOs, AP)] × 2 [replicate] × 6 [storage time].
Physicochemical analysis
This study analysed the free fatty acid content (Ca 5a-40 AOCS 2017), pH using a digital pH meter (A326 Thermo Scientific, Orion, USA), and lactic acid of butter samples using methods of Simsek 2011. The peroxide value was determined using Asdagh and Pirsa (2020)'s method by mixing butter (2 g) with an acetic acid and chloroform solution, treated with potassium iodide and titrated with sodium thiosulfate. The TBARs values were determined using a modified method by Baştürk et al. (2018) by mixing butter (5 g) with TBARs reagent. TBARs values were determined by measuring absorbance at 533 nm against a blank using a spectrophotometer (Shimadzu 1200 UV/Vis, Japan) applying the MDA standard curve and expressed as mg MDA per kg of the butter sample.
Determination of vitamin E (α-tocopherol)
This study used a modified method of Górnaś et al. (2014) to determine α-tocopherol in LB samples. The butter samples (0.5 g) were melted, dissolved in n-heptane (25 mL) and vortexed using a top vortex (Heidolph, Germany). The supernatant was transferred to a volumetric flask, repeating the procedure twice. The combined supernatants were evaporated under a nitrogen stream and filtered through a 0.45 μm PVDF filter prior to HPLC analysis. Chromatographic separation was performed on a Shimadzu HPLC system (Shimadzu Corporation, Kyoto, Japan) that included a pump (LC-20AT), degasser (DGU14A), low-pressure gradient unit (FCV-10ALvp), system controller (CBM-20A), auto-injector (SIL-20 AC), column oven (CTO-10ASvp) and fluorescence detector (RF-10AXL). Analysis was performed on a Luna PFP column (250 mm × 4.6 mm, 5 μm) with a guard column (10 mm × 4.6 mm) (Phenomenex, Torrance, CA). The mobile phase was n-heptane/tetrahydrofuran (95/5) (v/v) at a flow rate of 1.5 mL/min, column oven temperature of 40°C and injection volume of 20 μL. The fluorescence detector was calibrated with an excitation of 295 nm and an emission of 330 nm. The identification of α-tocopherol was achieved by comparing the retention times of individual peaks in the chromatograms of the samples with those of the standard and expressed as ng per kg of the butter sample.
Determination of vitamin A (retinol) and β-carotene
This study measured vitamin A and β-carotene levels in LB samples using a modified method of Hulshof et al. (2006); Cakmakci et al. (2023). The process involved extracting 1 g of butter with 30 mL of tetrahydrofuran containing BHT (0.01% w/v), filtered using Whatman No. 54 adding 25 mL tetrahydrofuran and evaporating to dryness before saponification. The solutions were filtered (0.22 μm syringe filter) and 10 μl was injected into HPLC using a C18, silica, 250 × 4.6 mm column for separation. The detector wavelength was set to 325–450 nm for vitamin A and β-carotene respectively. The mobile phase was methanol–tetrahydrofuran containing BHT 98/2 (v/v) at an isocratic flow rate of 0.8 mL/min. The LC-20 AD Prominence HPLC system (Shimadzu Corporation, Kyoto, Japan) was equipped with an SPD-M20A UV detector, a SIL-20A HT autosampler, a CTO-20A column oven and a DGU-20A5 degasser. The identification of vitamin A and β-carotene was achieved by comparing the retention times of individual peaks in the chromatograms of the samples with those of the standard and expressed as μg per kg of the butter sample.
Arrhenius kinetic model
The kinetic reaction order was determined by comparing the correlation coefficients of experimental temperatures (70°C, 80°C and 90°C), where C0 is the initial concentration, C is concentration after heating or storage and k is the reaction rate constant (meq O2/kg/s and mg MDA/kg/s, and s−1 for n = 0 and 1 respectively), and t is the heating or storage time (s or day) (Piedrahita et al. 2015; Akyildiz et al. 2021).
The reaction rate constant (k), pre-exponential factor (A), activation energy (Ea) J/mol, universal gas constant (R) J/mol/K and absolute temperature (T) K = 273+°C are all crucial factors in chemical reactions, with Ea determined through linear regression analysis of ln ki and 1/Ti (i = 70°C, 80°C and 90°C).
Statistical analysis
This study analysed lactic butter samples using Excel 2010 (Microsoft Corp., USA) and ANOVA, correlation test and discriminant analysis using XLSTAT (2021.4.1.1215, Addinsoft, USA). The ANOVA test determined significant differences between groups, while the correlation test examined the relationship between heat treatments, lipid oxidation and vitamin degradation. Discriminant analysis assessed the effect of heat treatments and antioxidant effect of M. communis EOs in LB samples. All data were collected in duplicate, and differences less than P < 0.05 were considered statistically significant.
RESULTS AND DISCUSSION
The properties of fresh and stored lactic butter samples
Table S1 (Supporting Information) reveals the properties of fresh lactic butter, made from heat-treated and cultured cream, with free fatty acidity levels ranging from 0.66 to 0.95 mg KOH/kg fat, lactic acid 0.09–0.21% and pH 4.67–4.93 respectively. Figure S1 (Supporting Information) shows the impact of thermal heating of the cream on the degradation of vitamins in fresh LB samples. The levels of vitamin A, α-tocopherol and β-carotene in the butter reduced with thermal heating temperature and time (Figure S1 in Supporting Information). The levels of vitamin A, α-tocopherol and β-carotene in the butter varied between 19.20 and 23.38 μg/g, 24.40 and 41.80 ng/g and 2.63 and 3.32 μg/g respectively (Table S1 in Supporting Information). Heating temperature (TE) and use of antioxidants (A) did not affect vitamin degradation, but heating time (TI) of the cream had a significant impact on the degradation of α-tocopherol (P < 0.05) in fresh LB samples as shown in ANOVA test in Table S2 (Supporting Information). This study supports previous findings that heating temperatures of the cream do not significantly affect fat-soluble vitamin contents in butter, milk and palm oil (Öktürk et al. 2001; Kuppithayanant et al. 2014; Bezie 2019). α-tocopherol degradation in butter can be significantly influenced by heating temperature and time of the cream, the ratio of oil surface area to volume and the type of oil used (Kuppithayanant et al. 2014; Kmiecik et al. 2019).
Figure S2 (Supporting Information) reveals the impact of distinct thermal heat treatments of the cream on PV and TBARs values of fresh LB samples. Butter typically develops off-flavours when the PV reaches 2 meq O2/kg of fat and the TBARs value exceeds 0.3 mg malonaldehyde/kg fat (Öktürk et al. 2001). The PV and TBARs values of fresh lactic butter ranges from 1.50 to 3.60 meq O2/kg fat and from 0.12 to 0.15 mg MDA/kg fat for CB, MCEOsB and APB samples (Figure S2 and Table S2 in Supporting Information). The results reveals that the stability of LB samples, measured by peroxide and malonaldehyde formation, is significantly influenced by the heating temperature and time at which the cream is treated (P < 0.05), (Table S2 in Supporting Information). The PV and TBARs results exhibited slight variations from prior research findings (Ozturk and Cakmakci 2006; Özkanli and Kaya 2007; Simsek 2011; Méndez-Cid et al. 2017). The oxidative stability of butter is also influenced by the composition of milk, cream manufacturing processes, type of butter, storage and variations in these aspects are expected between studies.
Butter quality undergoes notable alterations after being stored in refrigerator between 21 days and 90 days (Öktürk et al. 2001; El-Hajjaji et al. 2020; Flórez et al. 2022; Darmawan et al. 2023; Mansour and Sindi 2024). Figure S2 (Supporting Information) shows the impact of thermal heat treatments of the cream on PV and TBARs values of stored LB samples for 50 days at 4°C. The butter samples showed an increase in PV and TBARs values, ranging from 1.5 to 4.8 meq O2/kg fat and 0.1183–0.1606 mg MDA/kg fat, respectively, made from heat-treated cream at 70°C, 80°C and 90°C for 15, 30 and 60 s and stored in a refrigerator (Figure S2 in Supporting Information). PV and TBARs values of LB samples increased with the rate of lipid oxidation directly proportional to heating temperature and time of the cream, antioxidants use and storage period (P < 0.05) (Table S2 in Supporting Information).
Kinetic study of vitamin degradation of fresh lactic butter samples
Table 1 and Figure 2 display the kinetic data for vitamin degradation in fresh LB samples following different heat treatments.
| Parameters | Butter | Temperature (°C) | Regression equationa | R 2 | Vitamin degradation kinetics | ||||
|---|---|---|---|---|---|---|---|---|---|
| k (sˉ1 × 10ˉ3) | t 1/2 (s) | Q 10 | Ea (kJ/mol) | R 2 | |||||
| Vitamin A (Retinol) (μg/g fat) | CB | 70 | y = −0.0011x + 3.1685 | 0.9909 | 1.07 | 647.66 | |||
| 80 | y = −0.0017x + 3.1496 | 0.9790 | 1.70 | 407.65 | 1.41 | 35.74 | 0.9679 | ||
| 90 | y = −0.0021x + 3.1524 | 0.9233 | 2.13 | 325.35 | |||||
| MCEOsB | 70 | y = −0.0012x + 3.1683 | 0.9915 | 1.20 | 577.50 | ||||
| 80 | y = −0.0017x + 3.0669 | 0.9161 | 1.70 | 407.65 | 1.44 | 37.96 | 0.9979 | ||
| 90 | y = −0.0025x + 3.1199 | 0.9836 | 2.50 | 277.20 | |||||
| APB | 70 | y = −0.0016x + 3.1334 | 0.9637 | 1.60 | 433.13 | ||||
| 80 | y = −0.0020x + 3.1539 | 1.0000 | 2.00 | 346.50 | 1.50 | 41.79 | 0.9287 | ||
| 90 | y = −0.0036x + 3.1700 | 0.9969 | 3.60 | 192.50 | |||||
| α-tocopherol (ng/g fat) | CB | 70 | y = −0.0057x + 3.8053 | 0.9820 | 5.70 | 121.58 | |||
| 80 | y = −0.0066x + 3.5644 | 0.8282 | 6.60 | 105.00 | 1.08 | 8.36 | 0.8284 | ||
| 90 | y = −0.0067x + 3.7691 | 0.9706 | 6.70 | 103.43 | |||||
| MCEOsB | 70 | y = −0.0061x + 3.6507 | 0.9949 | 6.10 | 113.61 | ||||
| 80 | y = −0.0088x + 3.7153 | 0.8467 | 8.80 | 78.75 | 1.21 | 19.54 | 0.7825 | ||
| 90 | y = −0.0089x + 3.6967 | 0.9547 | 8.90 | 77.87 | |||||
| APB | 70 | y = −0.0051x + 3.7807 | 0.9459 | 5.10 | 135.88 | ||||
| 80 | y = −0.0068x + 3.7521 | 0.9688 | 6.80 | 101.91 | 1.33 | 29.19 | 1.0000 | ||
| 90 | y = −0.0090x + 3.7916 | 0.9983 | 9.00 | 77.00 | |||||
| β-carotene (μg/g fat) | CB | 70 | y = −0.0022x + 1.2397 | 0.9666 | 2.20 | 315.00 | |||
| 80 | y = −0.0029x + 1.2383 | 0.9978 | 2.90 | 238.97 | 1.17 | 16.03 | 0.8399 | ||
| 90 | y = −0.0030x + 1.1887 | 0.9664 | 3.00 | 231.00 | |||||
| MCEOsB | 70 | y = −0.0020x + 1.1883 | 0.9757 | 2.00 | 346.50 | ||||
| 80 | y = −0.0025x + 1.1744 | 0.8217 | 2.50 | 277.20 | 1.34 | 30.15 | 0.9776 | ||
| 90 | y = −0.0036x + 1.1803 | 0.9934 | 3.60 | 192.50 | |||||
| APB | 70 | y = −0.0018x + 1.2184 | 0.9646 | 1.84 | 376.63 | ||||
| 80 | y = −0.0026x + 1.2161 | 0.9978 | 2.56 | 270.70 | 1.36 | 31.27 | 0.9986 | ||
| 90 | y = −0.0034x + 1.2002 | 0.9486 | 3.38 | 205.03 | |||||
- a Linear regression equation of first-order reaction kinetics.
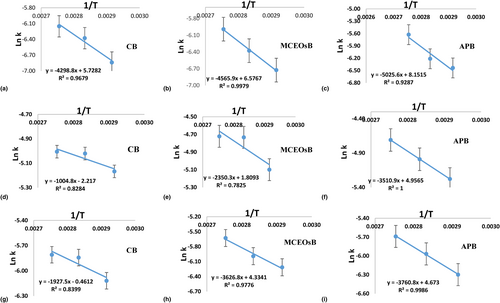
Kinetic models were established based on the rate constant of the reaction in freshly prepared LB samples (Table 1, Figure 2). The first-order reaction kinetics is suitable (since R2 is closest to 1) for the degradation of vitamin A, α-tocopherol and β-carotene in fresh LB samples. α-tocopherol exhibited the lowest Ea values of 8.36, 19.54 and 29.19 kJ/mol, for CB, MCEOsB and APB samples respectively. M. communis EOs and AP provide protection against vitamin degradation due to increased Ea values in lactic butter samples. However, LB samples containing M. communis EOs and AP had higher Q10 values, indicating that temperature has a greater impact on vitamin degradation in those lactic butter (Table 1).
Kinetic study of lipid oxidation of fresh lactic butter samples
Table 2 and Figure 3 present the kinetic information of the fresh LB samples after various heat treatments.
| Parameters | Butter | Temperature (°C) | Regression equation | R 2 | Lipid oxidation kineticsa | ||||
|---|---|---|---|---|---|---|---|---|---|
| k (meqO2/kg/s × 10−3) k (mgMDA/kg/s × 10−4) | t2 (s) | Q 10 | Ea (kJ/mol) | R 2 | |||||
| PV value (meq O2/kg fat) | CB | 70 | y = 0.0106x + 1.430 | 0.8104 | 10.60 | 141.51 | |||
| 80 | y = 0.0148x + 1.750 | 0.9276 | 14.80 | 128.38 | 1.06 | 32.63 | 0.9997 | ||
| 90 | y = 0.0200x + 2.400 | 0.7500 | 20.00 | 125.00 | |||||
| MCEOsB | 70 | y = 0.0089x + 1.483 | 0.9558 | 8.90 | 178.01 | ||||
| 80 | y = 0.0131x + 1.825 | 0.7772 | 13.10 | 145.04 | 1.16 | 38.71 | 1 | ||
| 90 | y = 0.0188x + 2.525 | 0.7121 | 18.80 | 138.30 | |||||
| APB | 70 | y = 0.0098x + 1.406 | 0.9093 | 9.80 | 153.06 | ||||
| 80 | y = 0.0143x + 1.750 | 0.9967 | 14.30 | 136.36 | 1.16 | 42.05 | 0.9967 | ||
| 90 | y = 0.0221x + 2.383 | 0.7608 | 22.10 | 113.12 | |||||
| TBARs value (mg MDA/kg fat) | CB | 70 | y = 0.00025x + 0.12000 | 0.9958 | 2.50 | 494.00 | |||
| 80 | y = 0.00029x + 0.12385 | 0.9835 | 2.90 | 439.66 | 1.09 | 11.17 | 0.9607 | ||
| 90 | y = 0.00031x + 0.12675 | 0.9713 | 3.10 | 420.97 | |||||
| MCEOsB | 70 | y = 0.00031x + 0.11757 | 0.9994 | 3.10 | 394.55 | ||||
| 80 | y = 0.00035x + 0.12205 | 0.9685 | 3.50 | 360.72 | 1.15 | 16.89 | 0.9732 | ||
| 90 | y = 0.00043x + 0.12329 | 0.9999 | 4.30 | 301.73 | |||||
| APB | 70 | y = 0.00034x + 0.11399 | 0.9828 | 3.40 | 348.03 | ||||
| 80 | y = 0.00042x + 0.11675 | 0.9719 | 4.20 | 290.10 | 1.18 | 20.99 | 0.9999 | ||
| 90 | y = 0.00051x + 0.11751 | 0.9864 | 5.10 | 243.34 | |||||
- a Kinetic data were calculated according to the zero-order reaction kinetics.
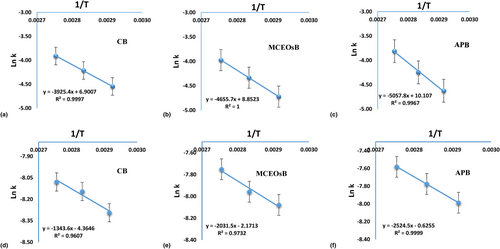
The formation of PV and TBARs in fresh lactic butter is most accurately described by zero-order formation kinetics at processing temperatures of 70°C, 80°C and 90°C for CB, MCEOsB and APB samples (Table 2, Figure 3). The results align with the study of Park et al. (2014), revealing that the appearance and pH of butter follow zero-order reaction kinetics. The activation energies for CB sample were 32.63 and 11.17 kJ/mol, while MCEOsB and APB samples were 38.71 and 16.89 kJ/mol and 42.05 and 20.99 kJ/mol for PV and TBARs respectively (Table 2). Lipid oxidation is easily initiated in the CB samples due to low Ea values. M. communis EOs and AP as antioxidants offer superior protection against lipid oxidation due to increased Ea values. However, the Q10 value of LB samples containing antioxidants is higher than the control (see Table 2), indicating a temperature dependence of lipid oxidation (Piedrahita et al. 2015; Jaimez-Ordaz et al. 2019), particularly in lactic MCEOsB and APB butter samples (Table 2). Ea values for butter and choibá oil were found to be either lower or similar to this study (Piedrahita et al. 2015; Basheer and Muthusamy 2022). Darmawan et al. (2023) found that lignin increased reaction rates and Q10 values in tengkawang butter.
The Pearson correlation test shows heating temperature of the cream affects PV and TBARs values (0.925, 0.480), while heating time of the cream affects the degradation of lipid soluble vitamin A, α-tocopherol and β-carotene (−0.664, −0.798 and −0.799), respectively, in fresh lactic butter samples (Table S3 in Supporting Information).
Kinetic study of lipid oxidation of stored lactic butter samples
Table 3 presents the average R2 values of the stored LB samples using Arrhenius parameters.
| Parameters | Lactic butter | Temp. (°C) | Time (s) | Lipid oxidation kinetics | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 15 s | 30 s | 60 s | ||||||||
| Regression equationa | R 2 | Regression equationa | R 2 | Regression equationa | R 2 | ks (dayˉ1 × 10ˉ3) | t2 (day) | |||
| PV value (meq O2/kg fat) | CB | 70 | y = 0.0086x + 0.4163 | 0.9634 | y = 0.0051x + 0.6014 | 0.9316 | y = 0.0069x + 0.7924 | 0.8122 | 8.17 | 91 |
| 80 | y = 0.0125x + 0.7047 | 0.9428 | y = 0.0089x + 0.8096 | 0.9534 | y = 0.0095x + 0.9159 | 0.9481 | ||||
| 90 | y = 0.0091x + 0.9763 | 0.9190 | y = 0.0071x + 1.1793 | 0.9738 | y = 0.0057x + 1.2814 | 0.9585 | ||||
| MCEOsB | 70 | y = 0.0055x + 0.4624 | 0.9605 | y = 0.0037x + 0.5716 | 0.9465 | y = 0.0072x + 0.7278 | 0.9526 | 5.88 | 128 | |
| 80 | y = 0.0089x + 0.6756 | 0.9836 | y = 0.0052x + 0.8600 | 0.9401 | y = 0.0069x + 0.9279 | 0.9875 | ||||
| 90 | y = 0.0072x + 0.9785 | 0.9777 | y = 0.0042x + 1.2224 | 0.9628 | y = 0.0041x + 1.2798 | 0.9839 | ||||
| APB | 70 | y = 0.0057x + 0.4112 | 0.9982 | y = 0.0033x + 0.5726 | 0.9597 | y = 0.0053x + 0.6668 | 0.9861 | 5.44 | 144 | |
| 80 | y = 0.0084x + 0.6499 | 0.9652 | y = 0.0062x + 0.7687 | 0.9568 | y = 0.0059x + 0.9377 | 0.9476 | ||||
| 90 | y = 0.0078x + 0.9402 | 0.9565 | y = 0.0035x + 1.2040 | 0.9806 | y = 0.0029x + 1.2841 | 0.9726 | ||||
| TBARs value (mg MDA/kg fat) | CB | 70 | y = 0.0024x-2.0889 | 0.9528 | y = 0.0024x-2.0391 | 0.9232 | y = 0.0023x-2.0025 | 0.9773 | 2.34 | 309 |
| 80 | y = 0.0023x-2.0554 | 0.9911 | y = 0.0024x-2.0232 | 0.9611 | y = 0.0020x-1.9747 | 0.9362 | ||||
| 90 | y = 0.0031x-2.0364 | 0.9727 | y = 0.0022x-1.9952 | 0.9598 | y = 0.0019x-1.9299 | 0.9560 | ||||
| MCEOsB | 70 | y = 0.0021x-2.0970 | 0.9836 | y = 0.0017x-2.0650 | 0.8860 | y = 0.0018x-2.0031 | 0.9512 | 1.73 | 470 | |
| 80 | y = 0.0013x-2.0730 | 0.9748 | y = 0.0023x-2.0074 | 0.9708 | y = 0.0012x-1.9499 | 0.9856 | ||||
| 90 | y = 0.0026x-2.0355 | 0.9781 | y = 0.0022x-2.0004 | 0.9481 | y = 0.0008x-1.9032 | 0.9732 | ||||
| APB | 70 | y = 0.0021x-2.1347 | 0.9756 | y = 0.0019x-2.0758 | 0.9318 | y = 0.0014x-2.0095 | 0.9549 | 1.91 | 447 | |
| 80 | y = 0.0018x-2.0981 | 0.9650 | y = 0.0023x-2.0292 | 0.9759 | y = 0.0012x-1.9634 | 0.9237 | ||||
| 90 | y = 0.0029x-2.0690 | 0.8429 | y = 0.0025x-2.0206 | 0.9507 | y = 0.0009x-1.9089 | 0.9387 | ||||
- a Linear regression equation of first-order reaction kinetics.
This study reveals that storage of lactic butter can lead to lipid oxidation which can be predicted using kinetic parameters. PV and TBARs formations in stored LB samples during storage can be adequately described by first-order reaction kinetics (Table 3). This study suggests that heat treatments of the cream at 70°C or 80°C for 30 s and storage at 4°C is recommended for reduced lipid oxidation rate in LB (Table 3). The average k values for PV and TBARs formation in stored butter samples CB, MCEOsB and APB were determined as follows: 8.17–5.88–5.44 and 2.34–1.73–1.91 × 10−3 days−1 respectively. M. communis EOs and AP reduce the reaction rate as evidenced by their lower k values compared with control LB samples during storage (P ≥ 0.05) (Table 3). The results align with previous research demonstrating the efficacy of M. communis EOs as an antioxidant (Aleksic and Knezevic 2014; Snoussi et al. 2021). This study also supports previous studies indicating that adding tomato by-product extracts, Artemisia and sandalwood EOs can decrease lipid oxidation rate in butter (Abid et al. 2017; Flórez et al. 2022; Boulares et al. 2023).
The ANOVA test shows that heating temperature and time of the cream, use of antioxidants and storage time significantly influence lipid oxidation of stored LB samples (P < 0.05) (Table S2 in Supporting Information). Pearson correlation test clearly indicates PV is mainly affected by heating temperature (0.837) while TBARs is affected by heating time (0.615) of the cream in stored lactic butter samples (Table S4 in Supporting Information).
Classification of lactic butter samples by discriminant analysis
Discriminant analysis classified LB samples from cultured cream treated with unique thermal heat treatments (70°C, 80°C and 90°C for 15, 30 and 60 s) and antioxidants (M. communis EOs and AP), as shown in Figure 4a–d.
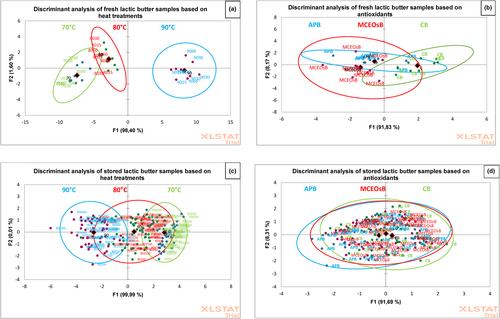
This study classified fresh and stored lactic butter samples using DA with an explanation of the total variance at 100%. The oxidative stability of lactic butter was significantly influenced by heating temperature, time, use of antioxidants and storage time (P < 0.05). Heat treatments of the cream significantly affected fresh lactic butter stability (Figure 4a). Fresh LB samples derived from the cream heat treated at 90°C exhibited notable distinctions compared with the samples treated at 70°C and 80°C (P < 0.05). The inclusion of M. communis EOs and AP significantly improved the oxidative stability of the fresh lactic butter (Figure 4b) but slightly improved in stored lactic butter samples (P ≥ 0.05) (Figure 4c,d). To improve lactic butter's oxidative stability and shelf life, cream should be heat treated at 70–80°C for 30 s and enriched with antioxidants like M. communis EOs.
This study shows that heat treatments have a significant effect on the stability of butter and that antioxidant activity is achieved at 200 ppm, in contrast to previous studies on butter oil, butter milk and butter which were carried out with antioxidant concentrations as low as 50 ppm (Ozturk and Cakmakci 2006; Özkanli and Kaya 2007; Guggisberg et al. 2012). M. communis EOs and AP as antioxidants were effective in reducing lipid oxidation and vitamin degradation in fresh lactic butter (P ≤ 0.05) but not in stored butter samples (P ≥ 0.05). This study's findings differ from previous ones due to the significant impact of milk or cream type, heating temperature of cream, type of butter, amount of antioxidant used and storage time on the stability of butter.
CONCLUSIONS
This study found that oxidation and degradation kinetics of lactic butter samples are influenced by heat treatments of cream and use of antioxidants. Lipid oxidation followed zero-order reaction kinetics in fresh lactic butter, whereas vitamin degradation in fresh lactic butter and lipid oxidation in stored lactic butter followed first-order reaction kinetics. Lactic butter samples are discriminated according to heat treatments and antioxidants by discriminant analysis. It is suggested that low-temperature heat treatments (70°C or 80°C for 30 s) are crucial to prevent lipid oxidation and α-tocopherol degradation during lactic butter production. M. communis EOs, is a natural, conceivable alternative to synthetic antioxidant ascorbyl palmitate, were found to reduce lipid oxidation and lipid soluble vitamin degradations in lactic butter during manufacturing and storage. This study will offer crucial insights into the production and quality of lactic butter, providing valuable insights for the dairy industry.
ACKNOWLEDGEMENTS
The authors sincerely thank the Cukurova University for financial support (Project number: 2019/11899) and Dr. Erdal Agcam for his technical and Dr. Merve Darici for her statistical assistance.
AUTHOR CONTRIBUTIONS
Tuba Simsek Mertoglu: Conceptualization; formal analysis; resources; methodology; visualization; writing – original draft; writing – review and editing. Turkan Mutlu Keceli: Conceptualization; methodology; software; data curation; supervision; formal analysis; writing – original draft; writing – review and editing; visualization; validation; resources.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study can be made available by the corresponding author upon request.




