Comparison of quality properties of pasteurized milk-based camel cheese depending on calf rennet concentration and microbial transglutaminase
Abstract
Research was conducted to investigate if ripened cheese can be manufactured from pasteurized camel milk with calf rennet. A further aim was to examine the texture modifying effect of microbial transglutaminase at different rennet concentrations. Ripened cheese samples were analysed for physical–chemical characteristics including colour, texture and essential amino acids. Results revealed that it is possible to make semi-hard camel cheese with the use of calf rennet. However, low-rennet dosage (2.94 IMCU/L) resulted in good textural properties (hardness, springiness) when commercial microbial transglutaminase was used.
INTRODUCTION
Camel-related research is ongoing worldwide, most papers are published from countries of the Near East, followed by India and Pakistan, and then by the United States of America (USA) and Australia, even research is arising from Europe (Iglesias et al. 2020). As a result of that, many scientists are working on the development of cheese products which may also be a solution to overcome the geographical distance and provide the benefits of camel milk to customers living far from the countries of origin. However, all researchers of camel cheese science are faced with the same hurdle, namely the structure and ratio of casein and whey proteins of camel milk, which are unfavourable for curdling and therefore effective cheese production (Farah and Ruegg 1989; Bornaz et al. 2009). The way to overcome this problem is diverse, with the majority of scientists applying camel chymosin (Bekele et al. 2018; Hailu et al. 2018) or plant extracts to substitute commercial rennet (Fguiri et al. 2021). Moreover, scientists tried to overcome the hurdles of cheese making with blends of camel's and cows' milk (Ayyash et al. 2021; Mbye et al. 2021a). Microbial transglutaminase (EC2.3.2.13) was already successfully applied for texture improvement by ripened cheese made of cow milk (Kuraishi et al. 1997; Di Pierro et al. 2010), buffalo milk (Darnay et al. 2022a), goat milk (Güzeler et al. 2020) due to its protein crosslinking action by acid-transfer reaction. So far, no scientists have tried different rennet concentration levels in the same study for the given cheese-making procedure. Moreover, most scientific papers based on camel milk are publishing results regarding only selected cheese categories such as soft cheese types (Mbye et al. 2020; Fguiri et al. 2021), fresh cheese (Zubeir and Jabreel 2008; Mbye et al. 2021a,b) and soft white cheese (Bekele et al. 2018, Bouazizi et al. 2021). Therefore, only one single article could be found on research aimed to produce ripened cheese with camel chymosin, namely a Cheddar type (Li et al. 2022). Our research work aims to understand the effect of different rennet concentrations on the production of semi-hard camel cheese as well as the use of microbial transglutaminase (MTG) by applying a novel injection method to improve texture also considering the simultaneous effects of both rennet and MTG enzyme on cheese characteristics.
MATERIALS AND METHODS
Manufacturing process of semi-hard Trappist cheese
Semi-hard Trappist cheese was manufactured from 5 litres of pasteurized (72°C, 15 s) camel milk (fat: 2.69% ± 0.14, protein: 2.83% ± 0.10, lactose: 4.39% ± 0.27) which was measured by Lactoscan MCC WS (Milkotronic Ltd, Nova Zagora, Bulgaria) right after arrival from Germany (Kamelfarm Marquard, Hittingen, Germany), and no other analytical measurement were conducted. Cheese milk was heated to 30°C in a stainless steel double wall cheese vat with 10 L volume (Agrometál, Budapest, Hungary) and tempered for vat work. Different dosages of bovine rennet enzyme (BiaRia Ltd, Budapest, Hungary – 210 IMCU/mL = International Milk-Clotting Unit, low rennet: 2.94 IMCU/L high rennet: 14.7 IMCU/L) were added. Direct Vat Set (DVS) Trappist cheese culture (BiaRia Ltd., Budapest, Hungary) at 0.0034 w/v % concentration was used for inoculation. Manufacturing of Trappist cheese were according to previous study (Darnay et al. 2022a, 2022b) with the following modifications After 45 min of coagulation, the curd was cut into 2 cm grain, size and was put in 100 g plastic cheese form and salted and left for drainage for 24 h. In case of MTG treatment commercially available microbial transglutaminase enzyme preparations Probind CH (BDF Natural Ingredients, Girona, Spain) and Activa YG (Ajinomoto Foods Europe SAS, Mesnil-Saint-Nicaise, France) were applied in 0.6 U/g protein concentration in 10% watery solution. The applied MTG preparation both have lactose but differ in other carriers: milk proteins in Probind CH 2.0, whereas in Activa YG yeast extract, maltodextrin and safflower oil. The enzyme solutions were injected with 1.2 × 50 mm needles in the cheese curd. The fresh cheese samples were then packaged in polystyrene-polyvinylidene chloride-polyethylene (PS/PVDC/PE) high gas permeable Flexo FCC ripening foil (Kalle Hungária Ltd., Budapest, Hungary). Cheese ripening was managed in a maturation cabinet (Forcar, Villa Verucchio, Italy) at standard environmental conditions (T = 14°C, RH = 85%). Ripening time was defined according to the requirements applying for Trappist cheese in Hungary given by Codex Alimentarius Hungaricus (CAH Dairy products, 2004). Ripened camel cheese samples were taken out after 4 weeks-long ageing for physical, chemical and textural analysis. Experiment was based on the manufacturing procedure of Trappist cheese from pasteurized milk and designed according to the above mentioned two independent variables (rennet concentrations/low, high/, commercial MTG preparations/control, CH, YG/). Manufacturing was repeated twice thus the results are based on independent trials.
Measurement of cheese colour
Surface colour of cheese rind was measured with Konica Minolta CHROMA METER CHR-400 tristimulus colour measuring system (Konica Minolta Sensing Europe B.V., Nieuwegein, the Netherlands) with previously published method (Darnay et al. 2022a) used for ripened buffalo cheese. Each surface was measured with 10 parallels. Values of difference in sensation calculated as was used to show the difference in colour due to milk processing and/or rennet concentration and/or MTG enzyme treatment and/or heat treatment.
Characterization of physical–chemical properties of experimental camel cheese
Manual pH meter Testo 206 (Testo AG., Titisee-Neustadt, Germany) was used to determine the pH level of 4-weeks-long ripened cheeses. Protein content was measured according to MSZ EN ISO 8968-1:2014 with 0.05% measurement uncertainty. Essential amino acid measurement is detailed in a separate section. The dry matter measurement was executed with an MB 160 moisture analyser (VWR, Debrecen, Hungary) at 105°C until weight stabilized.
SDS-PAGE
Experimental cheese milk resulted cheeses were analysed with Sodium dodecyl-sulphate polyacrylamide gel electrophoresis (SDS-PAGE). Cheese samples were prepared for SDS-PAGE analysis followed by a previously published method (Mbye et al. 2021a). Gel electrophoresis was carried out according to our previous study (Darnay et al. 2021) with slight modifications. Resolving gel solution (15%) was prepared by mixing 6 mL 30% acrylamide/Bis Solution (29:1), 2.7 mL 2 M Tris HCl (pH 8.8), 75 μL of SDS solution 10% (w/v), 3.09 mL deionized water, 75 μL of 10% APS (ammonium persulphate) and 9 μL N, N, N′, N′-Tetramethylethylenediamine. Resolving stacking gel solution (6%) was prepared by mixing of 0.75 mL 30% acrylamide/Bis Solution (29:1, v/v), 495 μL 0.5 M Tris HCl (pH 6.8), 42 μL SDS solution 10% (w/v), 2.4 mL deionized water, 38 μL 10% APS and 5 μL TEMED. The gels were stained for 30 min with 0.2% Coomassie brilliant blue (R-250; Bio-Rad Hungary Ltd., Budapest, Hungary). The range of molecular standard (Precision Plus Protein All Blue Standards; Bio-Rad, Hercules, CA, USA) was 250–10 kDa. The stained gel images were captured using a Gel Doc XR+ System (Bio-Rad). The identification and the densities of the bands were quantified using Quantity One and Image Lab 6.1 software programs (Bio-Rad).
Essential amino acid determination
Camel cheese was prepared for amino acid analysis strictly following the previously reported procedure (Darnay et al. 2022b). The prepared sample was analysed with high-performance liquid chromatography (HPLC) Agilent 1260 Infinity LC equipment (Agilent, Santa Clara, CA, USA) according to Agilent Application Code 5991-5571EN (Agilent Technologies 2017) with slight modifications. Pre-treated sample was put on standard pH 6.00 and measured with L-norvalin, (Thermo Fisher Scientific, Shanghai, China) used as internal standard. Amino acids were prepared with derivation using ortho-phthaladehyde (Alfa Aesar Chemical Co., Ltd., Shanghai, China) and 9-fluorenylmethyl-chloroformate (Thermo Fisher Scientific). The separation was done with C18 (Agilent) stable phase using HPLC with diode array detector (Agilent) at 338 nm and fluorescence detector (Agilent) with excitation at 340 nm and emission at 450 nm.
Texture profile analysis
Texture properties of camel cheese samples were measured with TA.XTPlus (Stable Micro Systems, Godalming, UK) Texture Analyser using a 500 N load cell. The preparation of cheese samples and the measurement conditions were according to our previously published method (Darnay et al. 2019). Force-time deformation curves were evaluated with Texture Exponent 32 as the given software of TA.XTPlus. Hardness and springiness parameters were selected for data evaluation.
Statistical analysis
Statistical analysis was performed by the software SPSS Statistics (version 27, International Business Machines Corporation, Armonk, NY, USA). One-way ANOVA was used to detect significant effects (P < 0.05). Kolmogorov–Smirnov test was used to check the normality of residuals and the homogeneity of variances was tested by Levene's test. Since the requirements were fulfilled, results were further evaluated by Tukey HSD post hoc test (P = 0.05). The data presented in Figures and Tables are indicated as mean ± SD.
RESULTS AND DISCUSSION
Colour measurements
Colour attributes of cheese samples were defined according to the colour difference (ΔE), which is calculated from lightness, redness and yellowness (as the respective formula above) between the compared samples (Table 1). Our results showed that higher rennet dosage itself may result in visible colour changes after a 4 week ripening time. Formerly scientists checked objective colour analysis for camel milk, camel cheese whey (Baig et al. 2022) or fresh camel cheese (Mbye et al. 2020; Fguiri et al. 2021). Previous study also concluded that the type of renneting enzyme may have a major role already in fresh camel cheese, as they found kiwi extract more favourable for sensorial colour properties, than camel chymosin (Fguiri et al. 2021). Moreover, other scientists (Mbye et al. 2021a) realized higher yellowness values in case of applying Withania extract for renneting as by camel chymosin (50 IMCU/L). Moreover, according to our study, both MTG enzyme preparations led to recognizable colour change compared to control; however, Probind CH caused higher values for colour difference than Activa YG. This effect was more pronounced at high rennet addition. Although there was only slightly recognizable difference in ΔE* between Activa YG and Probind CH treated cheeses independent of rennet concentration, it was due to difference in values of redness and yellowness by Probind CH. This enzyme preparation had only milk proteins beside lactose, but the exact composition is not given by the manufacturer; therefore, further study is needed to identify the protein fractions of this MTG preparation and their role in colour development.
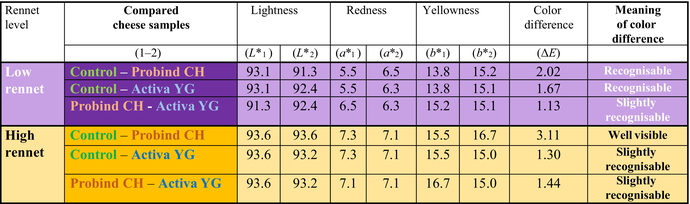
|
- Average values of lightness (L*), redness (a*) and yellowness (b*) are presented for the compared cheese sample (1: first, 2: second of compared cheese samples). Colour difference is calculated from the difference of lightness, redness and yellowness according to the given formula.
Physical–chemical properties of experimental camel cheese
Physical–chemical properties of ripened cheeses are summarized in Table 2. Cheese yields, which are results after 4 weeks long ripening of experimental cheeses, varied according to the applied rennet dosage and MTG activity. As the 4-weeks-long ripening was in high gas permeable ripening foil with 15 g/m2 water vapour permeability per hour, this allowed the building of cheese rind and drying, which led to 37% and 28% weight loss by Low Rennet and High Rennet respectively (data not presented). The lower the rennet dosage the higher the cheese yield was. The cheese yield values were doubled by low rennet independent from MTG enzyme preparations. However, Probind CH caused better values than Activa YG at both rennet concentrations. The rennet enzyme dosage slightly influenced the pH level of the ripened camel cheeses. Both applied MTG preparations (Activa YG, Probind CH) resulted in decreased pH value independent of the rennet dosage. This spectacular effect of MTG was not yet published for camel cheese; and therefore, this phenomenon needs further investigation. Both MTG preparations led to 4–16% higher protein levels and therefore 7–13% higher dry matter levels depending on the applied rennet dosage. Our results show that at low-rennet level (2.94 IMCU/L), Probind CH had probably better protein incorporation efficiency, than Activa YG. This may be due to the difference in carriers, as Probind CH had milk protein, towards which MTG has very high substrate specificity. The higher dry matter content was in all cases due to the higher protein levels in MTG-treated cheese samples.
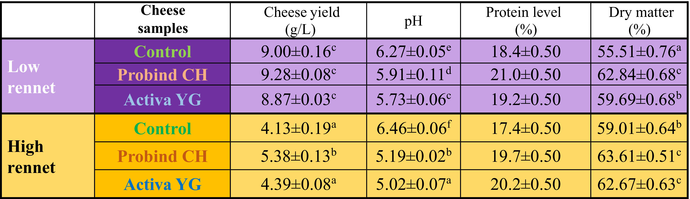
|
- Cheese yield, pH and dry matter content values are means ± SD. Protein level values are mean ± uncertainty. Means having different superscripts differ significantly (P < 0.05). Different colour shades are representing the highest concentration of different essential amino acids. Whereas highest concentratios are highlighted both among low rennet and high rennet experimental cheeses. As for eg histidine conectration was the highest at low rennet Activa YG and in case of high rennet experiments in case of high rennet Activa YG cheese.
SDS-PAGE
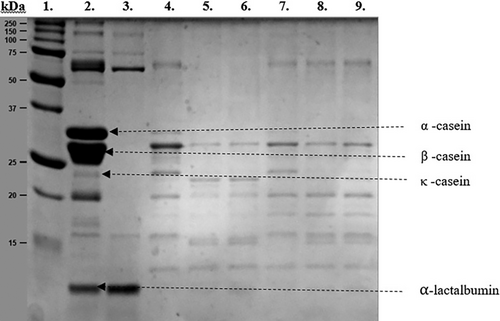
Figure 1 shows the peptide profiles of pasteurized camel milk and whey and cheeses made from pasteurized camel milk treated with MTG. The first column is the molecular standard, which allows the identification of proteins of 250–10 kDa. No significant difference can be observed between the two different molecular transglutaminases used, MTG-YG and MTG-CH, regardless of the concentration amounts used. The electrophoretic patterns of cheese proteins showed the appearance of several bands on the gel, differing in their migration position and the intensity of the bands. According to the band intensities, it can be concluded that the pasteurized camel milk used for cheese making had literally no κ-casein, but very high levels of β-casein, followed by α-casein and α-lactalbumin (Lane 2). Our gel pattern shows that among these protein fractions, α-lactalbumin was totally retained in the cheese whey (Lane 3) and therefore not present in any of the experimental cheeses (Lanes 4–9). This phenomenon was not reported before and needs further studies. In all cheese samples, it can be seen that casein was resolved into two main bands, αs–and β-caseins; however, the intensity of these bands was higher in the control than in the samples treated with MTGase (Abou-Soliman et al. 2020). The reduction and disappearance of casein band intensity indicates that these proteins are good substrates for the enzyme due to their flexible open structure (Monogioudi et al. 2009). MTG was unable to make high molecular weight polymers by cross-linking, which is different from the use of rennet at low concentrations (Lane 4). Here, new protein fractions with different molecular weights appeared in the lower molecular weight range in samples containing MTGase. The reason for this may be that the low MTG enzyme dosage (0.6 U/g protein) led to smaller peptides were formed, but they were not able to aggregate. Our gel electrophoretic analysis revealed that the both MTG enzyme preparations (Activa YG, Probind CH) are more effective with high rennet level (14.7 IMCU/L) as due to MTG more high molecular weight bands (60–65 kDa) appeared but also bovine rennet was able to produce low molecular weight bands (15–18 kDa) due to improved protein hydrolysis. This can be explained by the fact that MTGase is an acyltransferase that creates intra- and inter-molecular cross-links by forming isopeptide bonds between the lysine and glutamine groups of the peptide, resulting in cross-linking that promotes the formation of high molecular weight polymers (Abou-Soliman et al. 2020).
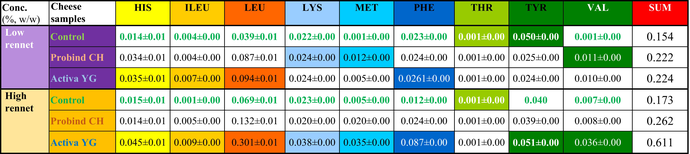
Essential amino acid analysis
Essential amino acid (EAA) profile of ripened camel cheeses is presented on Table 3. Control samples show the proteolytic effect of ripening after 4 weeks, which led to increased free amino acid (FAA) levels by high rennet dosage (14.7 IMCU/L) for leucine (+44%), methionine (+80%) and valine (+125%). Former study showed that MTG is only active at the day of cheese manufacturing (Darnay 2017); therefore, the concentration of available FAA is a result of previous cross-linking efficiency. As Probind CH enzyme preparation had additional milk proteins as carriers it could result in higher initial enzyme activity during the injection; however, the results show no significant difference to most of the EAAs at low-rennet dosage (2.94 IMCU/L). However, high-rennet dosage significant difference between Activa YG and Probind CH was recognized by all EAAs (TOP3: valine: +78%, histidine: + 69%, phenylalanine: 72%) which resulted to the highest concentration of EAAs (0.611%) among all experimental cheese types. This result is incomparable with any previous studies; therefore, more research is planned for better understanding.
|
- HIS, histidine; ILEU, ileucine; LEU, leucine; LYS, lysine; MET, methionine; PHE, phenylalanine; THR, threonine; TYR, tyrosine; VAL, valine.
- Different colour shades are representing the highest concentration of different essential amino acids. Whereas highest concentratios are highlighted both among low rennet and high rennet experimental cheeses. As for eg histidine conectration was the highest at low rennet Activa YG and in case of high rennet experiments in case of high rennet Activa YG cheese.
Texture profile analysis
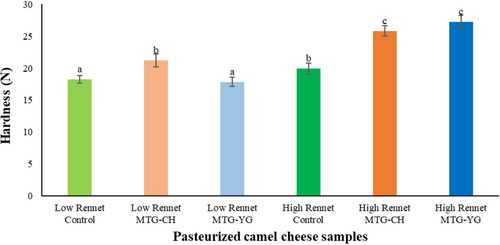
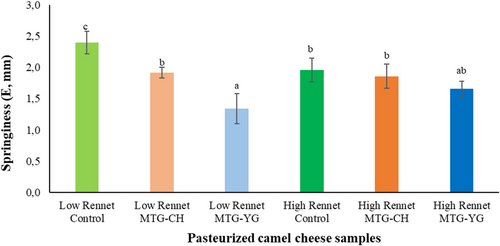
Hardness of cheese samples depended slightly on rennet addition as 8.5% difference was recognized between low-rennet control and high-rennet control on rennet enzyme dosage (Figure 2). Surprisingly, both MTG preparations could improve the hardness significantly at high rennet dosage (P < 0.05). However, at low-rennet dosage only Probind CH resulted in significant increase (P < 0.05) of hardness compared to control sample. According to our best knowledge, previous studies demonstrated hardness results only regarding soft cheeses. In comparison, these values were significantly (P < 0.05) lower (1–8 N) depending on different starter cultures (Bekele et al. 2018) or origin of camel milk (Mbye et al. 2021b). Recent publication also reflects the possible role of coagulants in texture improvement. Their study shows that camel chymosin (50 IMCU/L milk) results in higher hardness values in camel cheese than the use of 30% citric acid (Mbye et al. 2022). Results of springiness also reflect the effect of rennet on MTG preparations. Springiness significantly decreased (P < 0.05) only in control cheese samples resulting a negative correlation between the springiness and rennet enzyme dosage. Low-rennet concentration (2.94 IMCU/L) resulted in significant decrease (P < 0.05) of springiness by both MTG treated cheese samples; furthermore, there was a significant difference (P < 0.05) noticed between them (20.3% by Probind CH, 44.2% by Activa YG, see Figure 3). However, in the case of high rennet concentration (14.7 IMCU/L) both enzyme preparations had comparable springiness in cheese as control samples. According to these results, a simultaneous effect of rennet enzyme and microbial transglutaminase can be concluded as both harness and springiness values increased at High Rennet by both MTG-treated cheeses. This phenomenon needs reinvestigation in single phase model systems for better understanding and optimizing their cooperation.
CONCLUSIONS
In the present research, the combined effect of rennet and MTG was analysed on ripened cheeses made of pasteurized camel milk. Our study presents the importance of carefully selected rennet type and dosage as the physical–chemical characteristics and textural properties were greatly influenced. This research also wishes to highlight that various MTG preparations commercially available can affect camel milk properties not only based on their enzyme activity but also on the basis of different carriers. According to our study, the best suitable option is 2.94 IMCU/L and 0.6 U/g protein Probind CH.
ACKNOWLEDGEMENTS
The research was supported by Barentz Hungary Ltd, and BDF Ingredients, companies that benevolently provided us the commercial microbial transglutaminase enzyme preparations used in this study. Authors wish to thank BiaRia Ltd, who provided us with the bovine rennet enzyme and the cheese culture applied in this research. We wish to thank the Hungarian Dairy Research Institute for their analytical support and Anna Szepessy for her laboratory assistance.
AUTHOR CONTRIBUTIONS
Lívia Darnay: Conceptualization; investigation; project administration; writing – review and editing; writing – original draft. Annamária Barkó: Writing – original draft; methodology; validation; investigation. Karina Hidas: Data curation; formal analysis. Fanni Anna Pataki: Investigation; data curation. Gabriella Miklós: Methodology; validation; investigation. József Surányi: Resources. Péter Laczay: Resources; supervision.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




