The effect of soluble soybean polysaccharide addition to permeate concentrate on lactose crystallisation, growth and recovery during lactose manufacturing
Abstract
Lactose is manufactured from permeates of cheese whey, milk protein concentrate and a mix of both using a crystallisation process. Several factors, such as solute supersaturation, cooling rate and impurities, influence lactose crystallisation. This study focussed on the effect of soluble soybean polysaccharides (SSPS) on crystallisation during lactose manufacture from concentrated permeate solutions by evaluating lactose crystal growth and recovery with and without SSPS addition. Adding 0.1% (w/w) SSPS resulted in a significant difference (P > 0.05) in lactose crystal size and yield. The results suggest that using SSPS during lactose manufacturing from permeate concentrates could influence lactose crystallisation and recovery.
INTRODUCTION
Global cheese production is projected to grow at 1.51% (2.03% in the United States) by 2032, and an estimated 25.23 million tonnes of cheese was produced in 2022 (OECD/FAO 2023), corresponding to 227.1 million tonnes of cheese whey. The advancements in membrane filtration technology and the simultaneous increase in demand for high-protein dairy ingredients such as whey protein concentrates (WPC) and whey protein isolates (WPI) helped the industry to utilise large volumes of sweet whey produced, leaving behind a lactose-rich co-product stream, referred as whey permeate or deproteinised whey (DPW). Additionally, milk permeate is another lactose-rich co-product generated while manufacturing milk protein concentrate (MPC) and milk protein isolate (MPI). Newer products such as micellar casein concentrate manufactured using microfiltration also produce permeate rich in lactose (Marella et al. 2021; Hammam et al. 2022). Therefore, suboptimal utilisation of these co-products may limit the growth of mentioned high-protein dairy products and ingredients. Lactose is the principal component of milk and cheese whey permeates, comprising approximately 76–88% lactose, and forms the starting material in the lactose manufacturing process. Commercially, lactose is manufactured from concentrated permeate (65–75% total solids) by cooling and separating α-lactose monohydrate crystals (Wong et al. 2012). Because amorphous lactose creates processing (drying), post-processing (storage) and functionality issues, the concentrated lactose solution is usually crystallised before drying. Crystallisation of lactose in the supersaturated solution is initiated either by spontaneous crystal formation or by adding a small amount of lactose crystals for nucleation, often called seeding. The crystals are then separated using a decanting centrifuge with simultaneous washing by introducing wash water into the centrifuge. Adding wash water to remove mineral impurities is essential to producing high-quality lactose. The washed crystal slurry is then spray-dried and bagged. Crystallisation is a two-step process involving nucleation and crystal growth (Hartel 2001; Muthukumarappan et al. 2019), and the process objective of lactose crystallisation is to produce large crystals with a smaller number of fines to avoid loss of these fine particles during washing steps and achieve optimum yields (Pandalaneni and Amamcharla 2016; Paterson 2017).
Therefore, several studies were conducted to understand the factors affecting lactose crystallisation, for example, the presence of mineral contents (Jelen and Coulter 1973; Bhargava and Jelen 1996), different proteins and their degree of denaturation (Jelen et al. 1973; Modler and Lefkovitch 1986), pH levels (Modler and Lefkovitch 1986) and adding acids (Wijayasinghe et al. 2020). During lactose production, both nucleation and crystal growth stages are influenced by supersaturated conditions. Additives that can affect lactose solubility would alter the supersaturated conditions, thereby increasing the crystallisation rates. Adding alcohol (Majd and Nickerson 1976) and sucrose (Nickerson and Moore 1972), ultrasonication (Sutariya et al. 2018) reduced lactose solubility and promoted lactose crystallisation. It is a standard industry practice to add polysaccharides such as locus bean gum, guar gum, gelatin and xanthan gum as stabilisers in ice cream. These hydrocolloids increase the viscosity, thereby inhibiting the formation of ice and lactose crystals (Nickerson 1962). In another study, adding 0.9% κ-carrageenan delayed the crystallisation (Kouassi et al. 2002).
The soluble soybean polysaccharide (SSPS) is a cell wall extract of soybean cotyledon with the main chain composed of rhamnogalacturonan and homogalacturonan (Furuta and Maeda 1999), majorly consisting of acidic polysaccharide and small conjugated polypeptides fractions (Chivero et al. 2014), making it water-soluble. It is commercially obtained and refined from ‘okara’, the by-product of the soybean protein extraction process during the manufacture of soya protein isolate. The SSPS is functional as a dispersing agent, stabiliser, emulsifier and for its adhesion properties. The ability of SSPS to form a lower viscous solution compared with other polysaccharides enables its applications as a functional ingredient to stabilise proteins (Pan et al. 2014) and as a source of dietary fibre. The SSPS is a nongelling polysaccharide soluble in both cold and hot water, and it shows a relatively low viscosity compared with the viscosity of other gums or stabilisers, such as guar gum, allowing a highly concentrated (more than 30%) solution to be produced. Furthermore, the viscosity of the SSPS solution is not significantly affected by heating and the addition of acids or salts. The globular structure of SSPS can explain these physical properties in aqueous solution. Soyafibe-S is the most popular variety of SSPS and typically contains 60–77% soluble fibre, 4.8–13.9% crude protein and 6.0–8.6% ash. The constituents in Soyafibe-S are like pectin, acidic polysaccharides found in abundance in the peels of various fruits (e.g., citrus and apple). The predominant sugar components are galactose, arabinose and galacturonic acid, but many other sugars, such as rhamnose, fucose, xylose and glucose, are also present. However, the neutral monosaccharide content in SSPS is much higher than in pectin (Maeda and Nakamura 2009).
The influence of different SSPS levels on lactose crystal growth was conducted (Shi 2014) in an aqueous lactose solution, and a significant improvement in lactose recovery was reported. However, no information was available on the effect of SSPS on lactose crystallisation in concentrated permeates under industry-relevant conditions. Therefore, this study is focussed on evaluating the influence of SSPS addition to concentrated permeates while manufacturing lactose using industrially standard cooling rates (0.04°C/min.) and laboratory-scale crystallisers.
MATERIALS AND METHODS
Experimental design
The effect of SSPS on lactose crystallisation in concentrated permeates during lactose manufacture was studied at a 0.1% level of addition. The control (without SSPS) and treatment (with 0.1% w/w SSPS) concentrate permeates were crystallised using a 0.048°C/min cooling rate with 0.027% seed lactose crystals addition. All the experiments were carried out randomly and in triplicates, with control and treatments conducted simultaneously in a laboratory-scale crystalliser.
Concentrated permeate (CP) and soluble soybean polysaccharide (SSPS)
The CPs for the experiments were obtained from three different production lots of a nearby commercial dairy manufacturer (Valley Queen Cheese Factory, Milbank, SD). In addition, SSPS powder with 75% dietary fibre (w/w), a soluble fibre extracted and refined from soybean (Trade name, SOYAFIBE-S-CA100) used in the experiments, was donated by Fuji Oil Co., Ltd. (Osaka, Japan) as a research sample.
Lactose crystalliser set-up
A scaled-down version of the industry crystalliser was built to carry out the control and treatment experiments simultaneously, as shown in Figure 1. The crystalliser tanks consisted of two 1000-mL (105 mm × 142 mm, internal diameter (ID) × Height) jacketed borosilicate glass beakers with upper and lower 9.525 mm ID threaded hose connectors (Chemglass Life Sciences, Vineland, NJ) to circulate heating and cooling fluids through the jacket. A programmable refrigerated bath (Thermo Fisher Scientific, Newington, NH) generated a cooling medium to run desired crystallisation cooling rates connected to the jacketed glass beakers. The tank closure lids, caps and agitators with two sets of stirring arms and one bottom scrapping arm were printed using 3D printing technology (Falconplastics, Brookings, SD), as shown in Figure 1. Two overhead stirrers (RW 20 type, IKA Works, Inc. Wilmington, NC) were connected to each agitator to stir the concentrate permeate solutions in the tank.
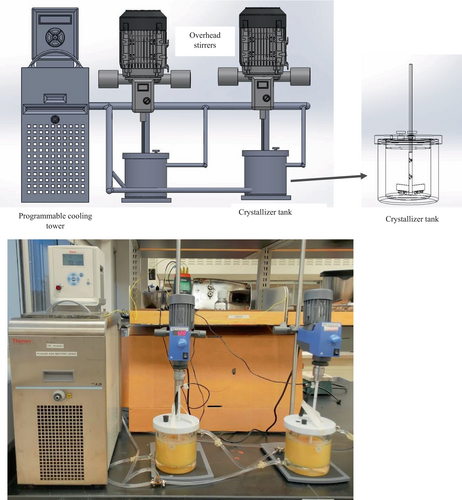
Lactose crystallisation process and recovery
Compositional analysis
Lactose content in concentrated permeates was determined using an HPLC system (Beckman Coulter Inc., Fullerton, CA) equipped with a solvent delivery module (System Gold 125), a multichannel wavelength scanning detector (190–600 nm; System Gold 168 detector) and a 20-μL sample injection loop (Rheodyne, Rohnert Park, CA) as described by Amamcharla and Metzger 2011. The total solid percentage was determined using oven drying (AOAC International 2002; method 990.20). Ash content was determined after ignition of the sample at 550°C (AOAC International 2002; method 954.46). All the supernatant collected from initial and successive washes from each control and treatment experiment was freeze-dried using the FreeZone 4.5 L freeze-dry system (Labconco Corporation, Kansas City, MO) to determine the SSPS content. The supernatant was preconcentrated using Hei-VAP Value laboratory vacuum evaporator (Heidolph Instruments GMbH andCo. KG) before freeze-drying to reduce the load on the freeze dryer and save time. The dried supernatant was analysed for SSPS using the Total Dietary Fiber Assay Kit (K-TDFR) supplied by Megazime International Ireland, based on AOAC Method 985.29.
Crystals characterisation
A series of qualitative analyses, including microscopy, thermography and spectroscopy, were used to characterise the lactose crystals thus obtained for crystal purity and growth.
Lactose crystal morphology
Composite lactose crystal slurry samples were observed using a polarised light microscope (model BX-53, Olympus America Inc., Center Valley, PA). The crystal images were captured with a camera (Olympus DP70) connected to the microscope. The length of crystals was measured using an image processor (Fuji ImageJ). The samples for capturing crystal images were prepared by placing the representative sample of crystal slurry at the centre of the microscopic slide, followed by placing a chilled water drop and spreading the crystals uniformly on the slide. The crystal images on different slide spots were captured for image processing. The longest side of the crystal along the tomahawk shape measured (μm) was expressed as crystal size and used to calculate crystal size distribution (CSD).
Differential scanning calorimetry (DSC)
The DSC measurements were carried out using a model Q1000 (TA instrument, New Caste, DE) to analyse thermal stability, crystallinity and glass transition temperature. The calorimeter was calibrated with indium at an onset temperature of 150°C. The powder lactose samples (5 ± 0.2 mg) were hermetically sealed in aluminium pans, and analysis was carried out under a flow of dry nitrogen at a temperature scan rate of 5°C/min. The samples were scanned from temperatures 20–180°C. The scans for pure lactose (Davisco Foods International, Inc., Le Sueur, MN), SSPS (Fuji Oil Co., Ltd., Oska, Japan) and lactose powders with and without SSPS were compared. The scans were processed and analysed using the Universal TA Instruments software V4.5A.
Fourier transform infrared spectroscopy (FTIR)
The FTIR spectra of dried lactose crystals were obtained in the range of 400–4000 cm−1 at a resolution of 4 cm−1 using a spectrometer (Cary 630, Agilent, Santa Clara, CA) with the OMNIC software (OMNIC V9.2, Thermo Fischer Scientific Inc.). Absorbance mode was used for the analysis after subtracting the background spectra. Each spectrum was an average of 32 scans, and all measurements were duplicated. The FTIR spectra of pure lactose (99.8% purity) obtained from Davisco Foods International, Inc, (Eden Prairie, MN), the SSPS (Fuji Oil Co., Ltd., Oska, Japan), lactose powders with and without SSPS, were also acquired, and compared with the dried lactose.
Statistical analysis
An independent sample t-test is performed to analyse the mean comparison of control and treatment triplicate data at a significance (P) value of 0.05 using the R (version 3.4.1) statistical program.
RESULTS AND DISCUSSION
Effect of lactose crystallisation and yield
Lactose crystal slurry obtained at the end of the crystallisation was corrected for the moisture content to enumerate the mass of α-lactose monohydrate crystals recovered. The lactose yield was calculated using Equation 1. The lactose recoveries without and with 0.1% w/w SSPS were in the range of 70.52–72.42% and 75.04–77.19%, respectively, as shown in Table 1. The crystallisation promotion effect of SSPS at 0.1% w/w level was significant (P < 0.05). Lactose exists in two anomeric forms, α- and β-lactose, which are converted to each other by muturation. In an unsaturated solution, lactose exists in soluble form surrounded by a hydration layer (Hartel 2001). As supersaturation conditions, such as concentration and cooling, are manipulated, a series of transformations between α- and β-lactose occurs. The added SSPS consisting of hydroxyl groups may show preferential hydration in CP and influence the degree of supersaturation conditions by reducing the solubility of lactose. The retention of part of the SSPS used in the treatment with the lactose crystals may explain the possible mechanism of SSPS servicing as the nucleation site and promoting secondary nucleation.
| Lactose crystal yield (%) | Ash (%) | SSPS retained in crystals (%) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |
| Without SSPS | 71.33 ± 0.98a | 70.52–72.42 | 1.33 ± 0.06a | 1.26–1.38 | – | – |
| With 0.1% SSPS | 76.31 ± 1.13b | 75.04–77.19 | 1.35 ± 0.05a | 1.29–1.38 | 20.18 ± 0.78 | 19.59–21.07 |
- SD, standard deviation; SSPS, soluble soybean polysachharide.
- Means values with different superscript are significantly different (P < 0.05).
Morphology and size distribution
The mean crystal size was significantly (P < 0.05) higher in the case of treatment (138.46 μm) than that of control (116.94 μm). The microscopic images of lactose crystals without SSPS and with 0.1% w/w SSPS are shown in Figure 2a,b, respectively. The preferential water binding capacity of added SSPS may have facilitated lactose–lactose interactions and promoted the growth of lactose crystals. Crystal size distribution of lactose, without and with SSPS, was compared in Figure 3. The crystal size distribution of crystals with SSPS was observed to be narrower and more centred towards bigger crystal sizes than those without SSPS. This can be correlated with the higher lactose crystal yield obtained. Lactose crystallisation and crystal properties are influenced by lactose and water interactions and can modulate the crystalline habits of lactose.
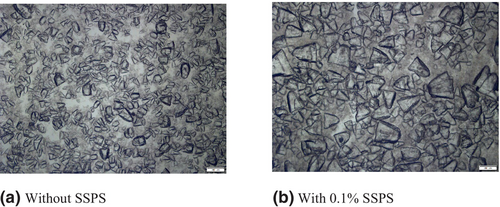
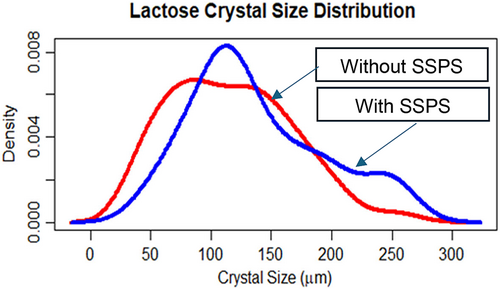
Lactose purity
The ash content in the washed crystal slurry was 1.26–1.38% and 1.29–1.38% (w/w) with and without 0.1% SSPS addition, respectively, as presented in Table 1. No significant change (P > 0.05) in the ash content was observed with the addition of SSPS. High ash content was observed in both control and treatment lactose crystals, and this may be due to the washing protocol used during the experiment being different from industry washing procedures.
The differential dietary fibre content between control and treatment wash water solutions was enumerated to calculate the SSPS content left in lactose crystal slurry. Out of the total SSPS added to the treatment solution, 79.82% was recovered into the wash water. This corresponds to 0.04% w/w of SSPS in lactose crystals. The impurities in the crystallising solution can result in morphological changes in lactose crystals because of the absorption on the surface of crystals.
Powder properties
The DSC curves of α-lactose monohydrate, SSPS and the lactose crystals recovered with and without SSPS addition are shown in Figure 4. The amorphous form of Lactose was identified by an exothermic peak at 167°C, representing the transformation of amorphous to crystalline form. The absence of this exothermic peak in the DSC curves of the lactose crystals recovered with and without SSPS suggests that there was no amorphous form of lactose present in these crystals. The endothermic peak in the case of α-lactose monohydrate at around 145°C represents the loss of crystalline water. The corresponding endothermic peaks of recovered lactose crystals from permeate appeared at around 147–149°C. This shift could be due to impurities such as minerals and proteins. Thomas et al. (2004) reported that crystal formation is favoured when the temperature is near the glass transition, but viscosity and diffusivity limit crystal growth. The remaining SSPS (0.04% w/w) in the lactose crystals treated with SSPS did not significantly affect the temperature of loss of crystalline water. This confirms that adding 0.1% of SSPS did not significantly affect the purity of the lactose crystals recovered from the permeate.
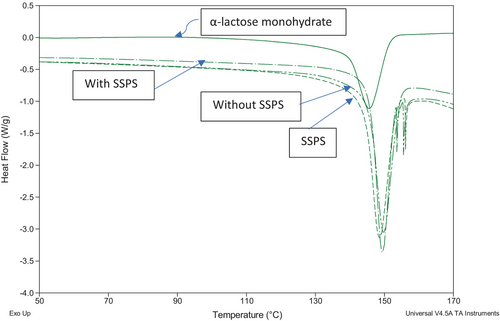
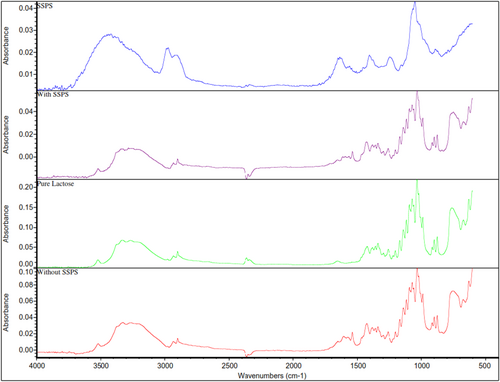
The FTIR spectra of SSPS, lactose crystals with SSPS addition, pure lactose monohydrate and lactose crystals without SSPS addition are presented in Figure 5. A specific peak at 1636 cm−1 in the spectra of SSPS corresponds to the amide groups, which was not present in the spectra of both control and treatment lactose crystals recovered. This indicates that no significant SSPS was present in the lactose crystals recovered with SSPS addition.
CONCLUSION
In this study, 0.1% (w/w) SSPS was added to the concentrate permeate to evaluate the commercial practicability of improving lactose crystallisation and recovery during lactose manufacture. The results suggest SSPS facilitates lactose crystal growth and promotes recovery from concentrated permeate solution. This could be due to the ability of SSPS to form low viscous solutions and to compete with lactose for water, thus positively influencing the degree of supersaturation of lactose. The addition of SSSP was observed to have no significant difference in the purity of the lactose crystals recovered. From the findings of this study, it is feasible to use SSPS to influence lactose crystal growth and recovery during the manufacture of lactose from concentrated permeated solutions.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Midwest Dairy Food Research Centre, SDSU, SD and Dairy Management Inc. (DMI), IL for financial support.
AUTHOR CONTRIBUTIONS
Venkateswarlu Sunkesula: Conceptualization; methodology; formal analysis; data curation; investigation; writing – original draft; writing – review and editing; software. Prafulla Salunke: Writing – review and editing; formal analysis; resources; validation; software. Lloyd E Metzger: Conceptualization; methodology; supervision; project administration; funding acquisition.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting this study's findings are available from the corresponding author upon reasonable request.




