Structure and cytotoxic properties of 1-hydroxy-5-methyl-7-phenylpyrido[3,4-d]pyridazin-4(3H)-one and its mono- and disubstituted ethyl acetates
Abstract
Derivatives of pyrido[3,4-d]pyridazine, namely, 1-hydroxy-5-methyl-7-phenylpyrido[3,4-d]pyridazin-4(3H)-one dimethylformamide monosolvate, C14H11N3O2·C3H7NO (2), ethyl [1-(2-ethoxy-2-oxoethoxy)-5-methyl-4-oxo-7-phenyl-3,4-dihydropyrido[3,4-d]pyridazin-3-yl]acetate, C18H17N3O4 (3), and ethyl [(5-methyl-4-oxo-7-phenyl-3,4-dihydropyrido[3,4-d]pyridazin-1-yl)oxy]acetate, C22H23N3O6 (4), were synthesized with the aim of discovering new potential biologically active agents. The properties of all three derivatives were characterized by 1H NMR, 13C NMR and FT–IR spectroscopic analysis. All the crystals were obtained by a solvent diffusion method from dimethylformamide (DMF) or dimethyl sulfoxide (DMSO) and characterized by single-crystal X-ray diffraction. The collected X-ray data revealed that the crystals of 2 and 4 belong to the triclinic space group P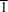 , whereas the crystal of 3 belongs to the monoclinic space group P21/c. The presented derivatives crystallized with one molecule in the asymmetric unit, but only compound 2 crystallized as a solvate with DMF. Structure analysis showed that the molecule of 2 exists as its amide–imidic acid tautomer and that O-alkylation occurred before N-alkylation during the synthesis of the mono- and disubstituted derivatives, i.e.3 and 4, respectively. The molecular geometries of the 5-methyl-7-phenylpyrido[3,4-d]pyridazine core within the studied derivatives differ in the mutual orientation of the rings. The interplanar angles between the heterocyclic ring and the bound aromatic ring are 1.71 (7), 18.16 (3) and 3.1 (1)° for 2, 3 and 4, respectively. The potential cytotoxicity of these compounds was evaluated against one normal (HaCat) and four human cancer cell lines (A549, DU145, MDA-MB-231 and SKOV-3).
, whereas the crystal of 3 belongs to the monoclinic space group P21/c. The presented derivatives crystallized with one molecule in the asymmetric unit, but only compound 2 crystallized as a solvate with DMF. Structure analysis showed that the molecule of 2 exists as its amide–imidic acid tautomer and that O-alkylation occurred before N-alkylation during the synthesis of the mono- and disubstituted derivatives, i.e.3 and 4, respectively. The molecular geometries of the 5-methyl-7-phenylpyrido[3,4-d]pyridazine core within the studied derivatives differ in the mutual orientation of the rings. The interplanar angles between the heterocyclic ring and the bound aromatic ring are 1.71 (7), 18.16 (3) and 3.1 (1)° for 2, 3 and 4, respectively. The potential cytotoxicity of these compounds was evaluated against one normal (HaCat) and four human cancer cell lines (A549, DU145, MDA-MB-231 and SKOV-3).




