Hydrogen-bonded supramolecular architecture in nonlinear optical ammonium 2,4-dinitrophenolate hydrate
Abstract
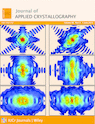
Single crystals of ammonium 2,4-dinitrophenolate hydrate (ADH) were grown by the slow evaporation solution growth technique. The structure is elucidated by single-crystal X-ray diffraction analysis and the crystal belongs to an orthorhombic system with noncentrosymmetric space group Pna21. The second harmonic generation efficiency of ADH is superior to that of the reference material KH2PO4. The X-ray study reveals that molecules are associated by weak C—H...O, O—H...N, N—H...π and π–π stacking interactions, which are responsible for the formation and strengthening of the supramolecular assembly. Inter- and intramolecular hydrogen-bonding interactions support the supramolecular architecture in the crystal packing. Three different types of architecture, i.e. column-like packing, a sandwich model of packing and a cluster network type of infrastructure, are observed. Optical studies reveal that the absorption is minimum in the visible region and the cutoff wavelength is at ∼240 nm. The band-gap energy was estimated by the application of the Kubelka–Munk algorithm. The powder X-ray diffraction pattern reveals the good crystallinity of the as-grown specimen. Investigation of the intermolecular interactions and crystal packing using Hirshfeld surface analysis, based on single-crystal X-ray diffraction, reveals that the close contacts are associated with molecular interactions. Fingerprint plots of Hirshfeld surfaces were used to locate and analyze the percentage of hydrogen-bonding interactions.