Redetermination of potassium chlorochromate, KCrO3Cl
Abstract
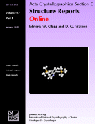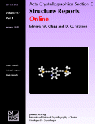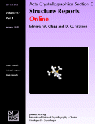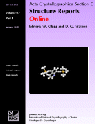
KCrO3Cl, potassium trioxochlorochromate(VI), has been redetermined from room-temperature single-crystal X-ray data. The Cr atom is tetrahedrally coordinated by three O atoms (average Cr—O bond length 1.597 Å) and one Cl atom at 2.1916 (8) Å, with nearly ideal tetrahedral angles. The K ion is coordinated by eight O and three Cl atoms. Isolated CrO3Cl tetrahedra and K ions are connected via common ligands. All atoms are in general positions.