Management of Hepatitis-B Virus Infection in Immunocompromised Children
A Single Center Experience
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
P.L.C. and M.P. are investigators in clinical trials sponsored by Gilead. The sponsors did neither play any role in the design, methods, data collection or analyses, nor did they do so in the preparation of the present article.
An infographic is available for this article at:http://links.lww.com/MPG/C161.
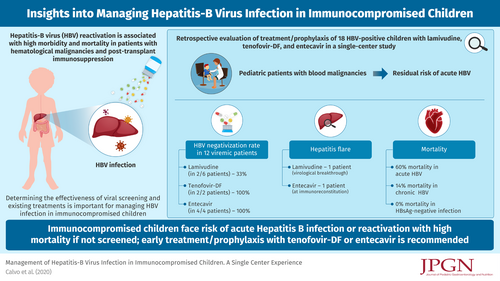
ABSTRACT
Objectives:
The aims of the study was to expand the pediatric experience on hepatitis-B virus (HBV) reactivation, a known complication in patients with hematologic malignancies or on immunosuppression.
Methods:
Retrospective appraisal of HBV therapy/prophylaxis in immunocompromised children, studied from April 2006 to March 2020.
Results:
Eighteen HBV-positive patients, 5 girls, median age 11.1 (4.1--17.9) years were included. Seventeen of 18 were immunosuppressed at HBV-infection diagnosis. Seventeen were at high risk of reactivation, 1 at moderate risk. Five of 18 had acute hepatitis B as first infection or reactivation, 6 had HBeAg-positive infection, 1 an HBeAg-negative infection and 6 HBsAg-negative infection. Median follow-up was 2.7 (0.7--12.5) years. No HBV-related mortality was observed. Prophylaxis had to be repeated in 1. Lamivudine was used in 6/12 viremic patients and HBV-DNA negativization obtained in 2/6 (33%). Tenofovir-DF was used in 2/12 and entecavir in 4/12: 100% attained HBV-DNA negativization. Therapy had to be switched from tenofovir-DF to entecavir in 1 patient because of renal impairment. Virological breakthroughs were observed in 1 lamivudine-treated patient, leading to a hepatitis flare; 1 patient on entecavir had a hepatitis flare at immunoreconstitution. Mortality was 33% in the HBsAg-positive group. Seven prophylactic treatments were administered to 6 patients with HBsAg-negative infection: tenofovir-DF in 2 HBV-DNA-positive, lamivudine in 5 HBV-DNA-negative, without reverse HBsAg seroconversion, morbidity or mortality.
Conclusions:
There is a residual risk of acute hepatitis B in immunocompromised children, mortality rate was substantial, potentially related to the delays in commencing chemotherapy caused by liver dysfunction. Tenofovir-DF or entecavir are the drugs of choice for HBV treatment in immunocompromised children.