Immune Response to Vaccination in Children and Young People With Inflammatory Bowel Disease
A Systematic Review and Meta-analysis
Corresponding Author
Łukasz Dembiński
Department of Pediatric Gastroenterology and Nutrition, The Medical University of Warsaw, Warsaw, Poland
Address correspondence and reprint requests to Łukasz Dembiński, MD, Department of Pediatric Gastroenterology and Nutrition, The Medical University of Warsaw, Żwirki i Wigury 63A, 02-091 Warsaw, Poland (e-mail: [email protected]).Search for more papers by this authorMarcin Dziekiewicz
Department of Pediatric Gastroenterology and Nutrition, The Medical University of Warsaw, Warsaw, Poland
Search for more papers by this authorAleksandra Banaszkiewicz
Department of Pediatric Gastroenterology and Nutrition, The Medical University of Warsaw, Warsaw, Poland
Search for more papers by this authorCorresponding Author
Łukasz Dembiński
Department of Pediatric Gastroenterology and Nutrition, The Medical University of Warsaw, Warsaw, Poland
Address correspondence and reprint requests to Łukasz Dembiński, MD, Department of Pediatric Gastroenterology and Nutrition, The Medical University of Warsaw, Żwirki i Wigury 63A, 02-091 Warsaw, Poland (e-mail: [email protected]).Search for more papers by this authorMarcin Dziekiewicz
Department of Pediatric Gastroenterology and Nutrition, The Medical University of Warsaw, Warsaw, Poland
Search for more papers by this authorAleksandra Banaszkiewicz
Department of Pediatric Gastroenterology and Nutrition, The Medical University of Warsaw, Warsaw, Poland
Search for more papers by this authorSupplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal's Web site (www.jpgn.org).
The authors report no conflicts of interest.
An infographic for this article is available at:http://links.lww.com/MPG/B863.
ABSTRACT
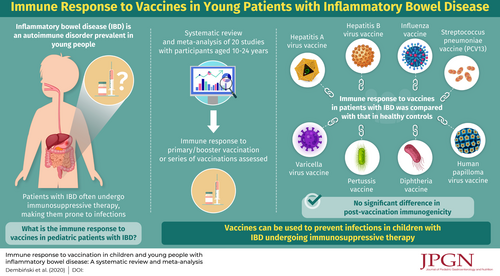
Objectives:
The aim of this systematic review and meta-analysis was to assess immune response to vaccination in children and young people with inflammatory bowel disease (IBD). In patients with IBDs, both the disease itself and its treatment can affect the vaccine response.
Methods:
Medical databases were searched for relevant studies and statistical analysis was performed. As a result, 20 publications were included in the study, 9 of which met the criteria for the meta-analysis.
Results:
The immune response to vaccination was better in healthy subjects (odds ratio = 0.73, 95% confidence interval = 0.45–1.17) and patients without immunosuppressive treatment (odds ratio = 0.65, 95% confidence interval = 0.41–1.03), but did not reach statistical significance.
Conclusions:
Immunogenicity of vaccinations in children and young people with IBD is not significantly lower than it is in healthy ones. Immune response to vaccination in this group of patients is also not significantly lower in patients on immunosuppressive therapy than in those without it.
Graphical Abstract
REFERENCES
- 1.Lakatos PL, Fischer S, Lakatos L, et al. Current concept on the pathogenesis of inflammatory bowel disease-crosstalk between genetic and microbial factors: pathogenic bacteria and altered bacterial sensing or changes in mucosal integrity take “toll”? World J Gastroenterol 2006; 12: 1829–1841.
- 2.Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015; 12: 720–727.
- 3.Ye Y, Manne S, Treem WR, et al. Prevalence of inflammatory bowel disease in pediatric and adult populations: recent estimates from large national databases in the United States, 2007-2016. Inflamm Bowel Dis 2020; 26: 619–625.
- 4.Roberts SE, Thorne K, Thapar N, et al. A systematic review and meta analysis of paediatric inflammatory bowel disease incidence and prevalence across Europe. J Crohns Colitis 2020; jjaa037.
- 5.Aberra FN, Lichtenstein GR. Methods to avoid infections in patients with inflammatory bowel disease. Inflamm Bowel Dis 2005; 11: 685–695.
- 6.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis 2014; 58: 309–318.
- 7.Memoli MJ, Athota R, Reed S, et al. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis 2014; 58: 214–224.
- 8.Floret N, Cervoni JP, Sheppard F, et al. Immunosuppression-mediated hepatitis B reactivation diagnosed following an investigation into suspected transfusion-transmitted hepatitis B. J Hosp Infect 2013; 83: 244–246.
- 9.Antonelli E, Baldoni M, Giovenali P, et al. Intestinal superinfections in patients with inflammatory bowel diseases. J Crohns Colitis 2012; 6: 154–159.
- 10.Rahier JF, Magro F, Abreu C, et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis 2014; 8: 443–468.
- 11.Veereman-Wauters G, De Ridder L, Veres G, et al. ESPGHAN IBD Porto Group. Risk of infection and prevention in pediatric patients with IBD: ESPGHAN IBD Porto Group commentary. J Pediatr Gastroenterol Nutr 2012; 54: 830–837.
- 12.Melmed GY, Agarwal N, Frenck RW, et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol 2010; 105: 148–154.
- 13. WHO. Recognizing adolescence. https://apps.who.int/adolescent/second-decade/section2/page1/recognizing-adolescence.html. Accessed March 16, 2020.
- 14.Phatak UP, Rojas-Velasquez D, Pashankar DS. Seroimmunity to hepatitis B virus in children with inflammatory bowel disease: effects of booster vaccination. J Pediatr Gastroenterol Nutr 2018; 66: e137.
- 15.Aljaberi N, Smitherman E, Watts A, et al. Protection against hepatitis B in immunocompromised pediatric rheumatology and gastroenterology patients. Pediatric Rheumatology 2018; 16: (suppl 2): 199.
- 16.DeBruyn J, Fonseca K, Ghosh S, et al. Immunogenicity of influenza vaccine for patients with inflammatory bowel disease on maintenance infliximab therapy: a randomized trial. Inflamm Bowel Dis 2016; 22: 638–647.
- 17.Fallahi G, Aghamohammadi A, Khodadad A, et al. Evaluation of antibody response to polysaccharide vaccine and switched memory B cells in pediatric patients with inflammatory bowel disease. Gut Liver 2014; 8: 24–28.
- 18.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097.
- 19.Lu Y, Bousvaros A. Varicella vaccination in children with inflammatory bowel disease receiving immunosuppressive therapy. J Pediatr Gastroenterol Nutr 2010; 50: 562–565.
- 20.Moses J, Alkhouri N, Shannon A, et al. Hepatitis B immunity and response to booster vaccination in children with inflammatory bowel disease treated with infliximab. Am J Gastroenterol 2012; 107: 133–138.
- 21.Elitsur Y, Preston D, Lopez-Marti M. Hepatitis B immunity and response to booster vaccination in WV children with inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: S91.
10.1097/01.MIB.0000438967.50619.c6 Google Scholar
- 22.Urganci N, Kalyoncu D. Immunogenecity of hepatitis A and B vaccination in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2013; 56: 412–415.
- 23.Phatak UP, Rojas-Velasquez D, Porto A, et al. Immune response to primary hepatitis B vaccination series in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2016; 63: S374.
- 24.Batra S, Tufano M, Diez B, et al. Comparison of hepatitis b booster versus hepatitis b immunization series administration and the effects on hepatitis b titer status in previously immunized inflammatory bowel disease patients undergoing biologic therapy. J Pediatr Gastroenterol Nutr 2017; 65: S282.
- 25.Brenner E, Jhaveri R, Kappelman M, et al. Evaluating hepatitis B seroprotection and revaccination for children with inflammatory bowel disease. Inflamm Bowel Dis 2019; 25: e108.
- 26.Radzikowski A, Banaszkiewicz A, Lazowska-Przeorek I, et al. Immunogenecity of hepatitis A vaccine in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2011; 17: 1117–1124.
- 27.Moses J, Alkhouri N, Shannon A, et al. Response to hepatitis A vaccine in children with inflammatory bowel disease receiving infliximab. Inflamm Bowel Dis 2011; 17: E160.
- 28.Mamula P, Markowitz JE, Piccoli DA, et al. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2007; 5: 851–856.
- 29.Lu Y, Jacobson DL, Ashworth LA, et al. Immune response to influenza vaccine in children with inflammatory bowel disease. Am J Gastroenterol 2009; 104: 444–453.
- 30.Romanowska M, Banaszkiewicz A, Nowak I, et al. Immunization against influenza during the 2005/2006 epidemic season and the humoral response in children with diagnosed inflammatory bowel disease (IBD). Med Sci Monit 2010; 16: RC433–RC439.
- 31.DeBruyn JC, Hilsden R, Fonseca K, et al. Immunogenicity and safety of influenza vaccination in children with inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 25–33.
- 32.Banaszkiewicz A, Targonska B, Kowalska-Duplaga K, et al. Immunogenicity of 13-valent pneumococcal conjugate vaccine in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis 2015; 21: 1607–1614.
- 33.Dembiński L, Krzesiek E, Klincewicz B, et al. Immunogenicity of diphtheria booster vaccination in adolescents with inflammatory bowel disease. Pediatr Infect Dis J 2020; 39: 244–246.
- 34.Jacobson DL, Bousvaros A, Ashworth L, et al. Immunogenicity and tolerability to human papillomavirus-like particle vaccine in girls and young women with inflammatory bowel disease. Inflamm Bowel Dis 2013; 19: 1441–1449.
- 35.Tung J, Kane S, Enders FT, et al. Hepatitis B vaccine in IBD. Am J Gastroenterol 2013; 108: 620–621.
- 36.Banaszkiewicz A, Gawronska A, Klincewicz B, et al. Immunogenicity of pertussis booster vaccination in adolescents with inflammatory bowel disease: a controlled study. J Pediatr Gastroenterol Nutr 2016; 62: 355.
- 37.Curtis L, Garrick V, McGrogan P, et al. Practicalities of varicella screening and vaccination in the paediatric inflammatory bowel disease (IBD) patients. J Crohns Colitis 2013; 7: S297.
10.1016/S1873-9946(13)60732-1 Google Scholar
- 38.Seitel T, Koletzko S, Krahl A, et al. Tolerability, safety, and efficacy of varicella-zoster-virus vaccination in immunosuppressed children with inflammatory bowel disease or autoimmune hepatitis. J Pediatr Gastroenterol Nutr 2019; 68: 72.
- 39.Nguyen DL, Nguyen ET, Bechtold ML. Effect of immunosuppressive therapies for the treatment of inflammatory bowel disease on response to routine vaccinations: a meta-analysis. Dig Dis Sci 2015; 60: 2446–2453.
- 40.Gisbert JP, Villagrasa JR, Rodriguez-Nogueiras A, et al. Efficacy of hepatitis B vaccination and revaccination and factors impacting on response in patients with inflammatory bowel disease. Am J Gastroenterol 2012; 107: 1460–1466.
- 41.Park SH, Yang SK, Park SK, et al. Efficacy of hepatitis A vaccination and factors impacting on seroconversion in patients with inflammatory bowel disease. Inflamm Bowel Dis 2014; 20: 69–74.
- 42.Cullen G, Bader C, Korzenik JR, et al. Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut 2012; 61: 385–391.
- 43.Neuzil KM, Jackson LA, Nelson J, et al. Immunogenicity and reactogenicity of 1 versus 2 doses of trivalent inactivated influenza vaccine in vaccine-naive 5-8-year-old children. J Infect Dis 2006; 194: 1032–1039.
- 44.Mugitani A, Ito K, Irie S, et al. Immunogenicity of the trivalent inactivated influenza vaccine in young children less than 4 years of age, with a focus on age and baseline antibodies. Clin Vaccine Immunol 2014; 21: 1253–1260.
- 45.Andrisani G, Frasca D, Romero M, et al. Immune response to influenza A/H1N1 vaccine in inflammatory bowel disease patients treated with anti TNF-alpha agents: effects of combined therapy with immunosuppressants. J Crohns Colitis 2013; 7: 301–307.
- 46.Camacho-Lovillo MS, Bulnes-Ramos A, Goycochea-Valdivia W, et al. Immunogenicity and safety of influenza vaccination in patients with juvenile idiopathic arthritis on biological therapy using the microneutralization assay. Pediatr Rheumatol Online J 2017; 15: 62.
- 47.Ogimi C, Tanaka R, Saitoh A, et al. Immunogenicity of influenza vaccine in children with pediatric rheumatic diseases receiving immunosuppressive agents. Pediatr Infect Dis J 2011; 30: 208–211.
- 48.Liao Z, Tang H, Xu X, et al. Immunogenicity and safety of influenza vaccination in systemic lupus erythematosus patients compared with healthy controls: a meta-analysis. PLoS One 2016; 11: e0147856.
- 49.Puges M, Biscay P, Barnetche T, et al. Immunogenicity and impact on disease activity of influenza and pneumococcal vaccines in systemic lupus erythematosus: a systematic literature review and meta-analysis. Rheumatology (Oxford) 2016; 55: 1664–1672.
- 50.Subesinghe S, Bechman K, Rutherford AI, et al. A systematic review and metaanalysis of antirheumatic drugs and vaccine immunogenicity in rheumatoid arthritis. J Rheumatol 2018; 45: 733–744.
- 51.Sousa S, Duarte AC, Cordeiro I, et al. Efficacy and safety of vaccination in pediatric patients with systemic inflammatory rheumatic diseases: a systematic review of the literature. Acta Reumatol Port 2017; 42: 8–16.